http://www.chemistrymag.org/cji/2000/021002pe.htm |
|
CO selective reduction of aromatic nitro compounds catalyzed by Ru3(CO)9(PETPP)3 in two-phase system
Mei Jianting, Jiang Jingyang,
Li Yaming, Jin Zilin
(State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116012,
China)
Abstract CO selective reduction of aromatic nitro compounds
catalyzed by Ru3(CO)9(PETPP)3 complex ( PETPP= P[C6H4-p-(OC2H4)nOH]3
) in aqueous/organic two-phase system was investigated. The catalyst displayed good
catalytic activity for the reduction of o-ClC6H4NO2 at
140ºC and Pco= 4 MPa : conversion of o-ClC6H4NO2
was 99.2% and selectivity toward the desired
amines was higher than 99%. After the reduction the catalyst in water phase could be
easily separated from the organic phase containing product and could be employed in the
successive reaction runs. The conversion of aromatic nitro compound could be kept higher
than 88% after 4 recycles of the catalyst.
Keywords Water-soluble phosphine ruthenium complex, Biphasic catalysis, Aromatic
nitro compounds, CO selective reduction, TRPTC
1. INTRODUCTION
Aromatic amines carrying carbonyl, chloro-, cyano and C=C groups are important
intermediates for the dye, pigment, pharmaceutical and pesticide industries [1].
Generally, these amines have been produced from the corresponding nitro compounds by the
reduction using iron powder, or alkali metal sulfides, or by catalytic hydrogenation.
However, the problem of treatment of the by-products and loss of the selectivity toward
the desired aromatic amines remains [2,3]. Recently it has been discovered that
reduction of aromatic nitro compounds to their corresponding aromatic amines using CO and
water with complex catalyst is an very attractive subject from the viewpoints of both high
chemoselectivity and environmental friendliness [4,5]. Tafesh [6]
studied CO selective reduction of aromatic nitro compounds in aqueous/organic biphasic
system catalyzed by water-soluble complex PdCl2/TPPTS (TPPTS = P(C6H4-3-SO3Na)3
). Under the conditions of 15MPa and 100ºC, the
conversion of the aromatic nitro compounds reached 70% with 15% of the by-products.
Jin et al proposed a conception of thermoregulated phase-transfer
catalysis (TRPTC) based on the nonionic water-soluble phosphine ligands, which possess an
inverse temperature-dependent solubility in water and thus a distinct cloud point.
Promising results have been achieved in the aqueous/organic two-phase hydroformylation of
higher olefins catalyzed by Rh/PETPP [7-9] . In this work Ru3(CO)9(PETPP)3
(PETPP = P[C6H4-p-(OC2H4)nOH]3
n = 6 ) was synthesized and applied in the CO selective reduction of aromatic nitro
compounds in the aqueous/organic two-phase system. Under the reaction conditions of 140ºC, Pco=4MPa, and t=10h, the conversion of o-ClC6H4NO2
was 99.2% and the selectivity of o-ClC6H4NH2 was
higher than 99%. The catalyst retained in water layer could be easily recovered and
employed successively. Furthermore, when the system was applied in the reaction of
aromatic nitro compounds containing C=O or C=N groups, the reduction proceeded with good
activity and high selectivity too.
2. EXPERIMENTAL
2.1 General
All solvents and liquid aromatic nitro compounds were distilled prior to use.
Distilled deionized water was used. Gas chromatographic analysis was performed on a SP-09
instrument (OV-101, 50m capillary column, carrier gas: 0.2MPa N2, FID detector)
equipped with a Shimadzu C-R3A integrator. n-Decane was used as an internal standard. IR
and 31P-NMR were recorded on a Nicolet 200X-BFT-IR spectrometer and on a
JEOLFX-90Q-NMR spectrometer separately. Mass spectra were measured on a Finnagan 312/ss
200 GC-mass spectrometer.
2.2 Catalysts preparation
PETPP, Ru3(CO)12 were prepared according to literature
[8-10]. Ru3(CO)9(PETPP)3 was prepared according to
the method for the preparation of Ru3(CO)9(TPPTS)3 [11,12].
The complex was characterized by IR and 31P-NMR(36.20MHz, internal standard
85%H3PO4, 45ºC, D2O).
The results were C=O(cm-1):2036(m),1972(m),1965(s); 31P-NMR:d=34.5ppm.
2.3 CO reduction
CO selective reductions of aromatic nitro compounds to aromatic amines were carried
out in a 75ml stainless steel autoclave, which was placed in a thermostatic oil bath. To
this were added toluene, water, aromatic nitro compounds, Ru3(CO)9(PETPP)3
and the internal standard n-decane. Then the autoclave was flushed five times with CO up
to the reaction pressure and held at the designated temperature with magnetic stirring for
a fixed time. After the completion of the reaction, the reactor was cooled and discharged
and the reaction solution was siphoned into a separatory funnel. After phase separation,
the organic layer was washed with distilled water, dried over anhydrous magnesium sulfate
and subjected to GC analysis.
3. RESULTS AND DISCUSSION
3.1 CO selective reduction of o-ClC6H4NO2
The data in Table 1 indicate that the rate of CO selective reduction increases with
the increase of reaction temperature and CO pressure. Higher than 99% of conversion of
o-ClC6H4NO2 and 99% of the yield of desired amine were
obtained when the reaction was carried out at 140ºC.
The recycling effect of the catalyst was also examined. Ru3(CO)9(PETPP)3
catalyst retained in aqueous phase after phase separation was used for 4 times with little
loss of the catalyst activity (entries 6-9).
Table 1 Two-phase CO selective reduction of o-ClC6H4NO2 catalyzed by Ru3(CO)9(PETPP)3 catalysts a
| Entry | Cycleb |
Tem/ºC |
P/PMa |
Time(h) |
Conv.(%) |
Yield(%) |
1 |
0 |
60 |
4 |
10 |
2.5 |
2.0 |
2 |
0 |
80 |
4 |
10 |
15.8 |
15.7 |
3 |
0 |
100 |
4 |
10 |
73.0 |
72.9 |
4 |
0 |
120 |
4 |
10 |
86.5 |
86.4 |
5 |
0 |
140 |
4 |
10 |
99.2 |
99.0 |
6 |
1 |
140 |
4 |
10 |
96.7 |
96.5 |
7 |
2 |
140 |
4 |
10 |
95.8 |
95.7 |
8 |
3 |
140 |
4 |
10 |
92.4 |
92.2 |
9 |
4 |
140 |
4 |
10 |
88.6 |
88.5 |
10 |
0 |
140 |
1 |
10 |
67.5 |
67.2 |
11 |
0 |
140 |
2 |
10 |
96.7 |
96.3 |
12 |
0 |
140 |
3 |
10 |
97.8 |
97.4 |
13 |
0 |
140 |
4 |
8 |
92.6 |
92.3 |
14 |
0 |
140 |
4 |
6 |
63.3 |
63.2 |
15 |
0 |
140 |
4 |
4 |
28.4 |
28.2 |
a: Reaction conditions:Ru3(CO)9(PETPP)3
=0.01mmol, V(toluene)/V(water)=4/4(ml),
o-ClC6H4NO2=10mmol.; b: Number of catalyst
recycles.
3.2 The CO selective reduction of aromatic nitro compounds carrying
other functional groups
Aromatic nitro compounds bearing other functional groups in CO selective reduction
catalyzed by Ru3(CO)9(PETPP)3 in water/organic biphasic
system were tested and the results were listed in Table2. The results showed that both the
chemoselective reduction of the nitro group and the yields of corresponding aromatic
amines are all higher than 98%.
Table 2 The CO selective reduction of some aromatic nitro compounds and aromatic compounds
| Reactant | Product |
Conversion(%) |
Yield(%) |
o-ClC6H4NO2 |
o-ClC6H4NH2 |
99.2 |
99.1 |
m-ClC6H4NO2 |
m-ClC6H4NH2 |
99.6 |
99.4 |
p-NO2C6H4CH2CN |
p-NH2C6H4CH2CN |
97.4 |
97.1 |
p-NO2C6H4COCH3 |
p-NH2C6H4COCH3 |
95.8 |
95.4 |
C6H5CHCH2 |
C6H5CH2CH3 |
No reaction |
|
C6H5CN |
C6H5CH2NH2 |
No reaction |
|
C6H5Br |
C6H6 |
No reaction |
|
C6H5CHO |
C6H5CH2OH |
0.06 |
0.03 |
Reaction conditions:T=140ºC, P(CO)=4Mpa,t=10hr,all other conditions are the same as in Table 1.
In order to affirm that the reduction reaction by CO
is chemoselective toward nitro group only, compounds with other reducible functional group
are chosen as substrate. Under the same reaction conditions, benzenenitrile, bromobenzene
and styrene do not react, and very little benzylalcohol from benzaldehyde was detected.
These data support that Ru3(CO)9(PETPP)3 is highly active
and chemoselective for nitro group as well. Such a high chemoselectivity can be explained
that the nitro group is not reduced by hydrogen from water-gas shift reaction. Instead it
has been postulated that the reduction aromatic nitro compounds involves a probable
ruthenium nitrene complex intermediate through successive oxygen transfer reaction between
the nitro compound and coordinated metal-carbonyl ligand [4]. A possible
mechanism is illustrated in Fig1.
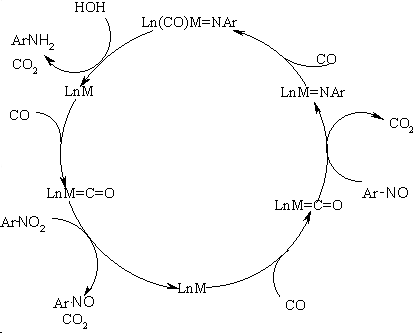
Fig.1 Mechanism of the CO selective
reduction of aromatic nitro compounds
3.3 CO selective reduction of o-ClC6H4NO2 catalyzed by
different Ru/P complexes
Four catalysts possessed different water-solubilities are
tested for the two-phase CO selective reduction of o-ClC6H4NO2
and the results are listed in Table 3. The data in Table 3 show that the catalyst activity
decreases as water-solubility of the ligand increases from TPP to TPPTS. Although the
water-solubility of the PETPP is nearly the same as that of the TPPTS, the catalytic
activity of the PETPP/Ru is the same as that of the water insoluble TPP/Ru. This
phenomenon could be explained from the thermoregulated phase-transfer function of Ru3(CO)9(PETPP)3
catalyst. The Ru3(CO)9(PETPP)3 used has a cloud point of
Tp = 82.5ºC and thus possesses a property of inverse temperature-dependent
water-solubility. That is, the Ru3(CO)9(PETPP)3 catalyst
soluble in the aqueous phase at lower temperature can transfer into organic phase to
catalyze the CO selective reduction of aromatic nitro compounds at temperature higher than
Tp. Therefore, CO selective reduction of o-ClC6H4NO2
occurs in the organic phase. The experimental results revealed that there was a 'thermoregulated phase-transfer catalysis (TRPTC)' process
present in the reaction.
Table 3 The activities of different phosphine/Ruthenium on the CO reduction of o-ClC6H4NO2
| Ligand | Water solubility |
Catalyst |
Conv.(%) |
Yield(%) |
TPP |
i.s |
Ru3(CO)9(TPP)3 |
99.4 |
99.2 |
TPPMS |
sl |
Ru3(CO)9(TPPMS)3 |
82.6 |
82.5 |
TPPTS |
vs |
Ru3(CO)9(TPPTS)3 |
38.5 |
38.3 |
PETPP |
vs |
Ru3(CO)9(PETPP)3 |
99.2 |
99.1 |
Reaction conditions: catalyst = 0.01mmol,T=140ºC, P(CO) = 4 Mpa, t = 10h. Other conditions are the same as Table 1.
REFERENCES
[1] Mestroni G, Camus A. Zassinovich in Aspects of Homogeneous Catalysis, 1981, 4:
71.
[2] Jenner G, Taleb B A. J. Mol. Catal., 1991, 68: 257.
[3] Nomura K. J. Mol. Catal. A. Chemical, 1998, 130: 14.
[4] Nomura K. J. Mol. Catal. A. Chemical, 1995, 95: 203.
[5] Tafesh A M, Weigun Y J. Chem. Rev., 1996, 96: 2035.
[6] Tafesh A, Beller M. Tetrhedron Lett., 1995, 36: 9305.
[7] Jin Z L, Zheng X L, Fell B. J. Mol. Catal., 1997, 116: 55.
[8] Zheng X L, Jiang J Y, Liu X Z, Jin Z L. Catalysis Today, 1998, 44:175.
[9] Chen R F, Liu X Z, Jin Z L. J.Organomet. Chem., 1998, 571: 201.
[10] Eady C R, Jackson P F, Johnson F G et al. J. Chem. Soc., Dalton Trans., 1980, 383.
[11] Fontal B, Orlewski J, Santini C C et al. Inorg. Chem., 1986, 25: 4320.
[12] Jin Z L, Zheng X L. Aqueous Phase Organometallic Catalysis -Concepts and Application.
Weinheim: Wiley-VCH, 1998: 233-240.