http://www.chemistrymag.org/cji/2000/02b049pe.htm |
Nov. 18, 2000 Vol.2 No.11 P.49 Copyright |
A voltammetric enzyme-linked immunoassay for southern bean mosaic virus
Shusheng Zhang, Kui Jiao
(Department of Applied Chemistry, Qingdao Institute of Chemical Technology, Qingdao,
266042, China)
Received Sep.1, 2000; Supported by the National Natural Science Foundation of China (No. 29775012) and the Youth Foundation of Shandong Province (Q99B16). This paper is one of the series studies of "Studies on the voltammetric enzyme-linked immunoassay".
Abstract A voltammetric enzyme-linked
immunoassay based on a system of o-aminophenol (OAP)-H2O2-horseradish
peroxidase (HRP) has been developed and used for the detection of HRP and southern bean
mosaic virus (SBMV). HRP or HRP labelled IgG (IgG-HRP) catalyzes the oxidation reaction of
OAP with H2O2, the product of which produces a sensitive
voltammetric peak at potential of -0.87 V (vs. SCE) in Britton-Robinson (BR) buffer
solution. By using this voltammetric peak, HRP can be measured with a detection limit of
3.5¡Á10-12 g/mL and a linear range of 6.0¡Á10-12-4.0¡Á10-9
g/mL. The SBMV has successfully been detected, and the satisfactory results have been
obtained.
Keywords Southern bean mosaic virus, o-Aminophenol, Horseradish peroxidase,
Voltammetry
Immunoassay with electrochemical detection is
an attractive method in bioanalytical chemistry due to its high sensitivity and low
detection limit. Technique with enzyme labels is by far mostly used in electrochemical
immunoassay. Heineman et al have applied alkaline phosphatase to the detection of some
antigens and drugs[1-5], most of which are involved in amperometric
enzyme-linked immunoassay. There are only a few documents on voltammetric enzyme-linked
immunoassay. Kui Jiao et al have developed the use of o-dianisidine[6] for
voltammetric detection of HRP, labelled HRP and some plant viruses.
In this paper, a system of OAP-H2O2-HRP
voltammetric enzyme-linked immunoassay has been advanced. Voltammetry is used to detect
the product of H2O2 oxidizing OAP, which is catalyzed by HRP. The
method has been successfully applied to the detection of southern bean mosaic virus
(SBMV), which has a number of plant strains and a wide spread hosts, and makes inroads on
hundreds of plants. The sensitive determination of SBMV in various plants and seeds is
very important[7,8]. The detecton limit to the purified SBMV with this method
is 8.0 ng /mL and the highest dilution ratio detected to the SBMV-infected leaf sap is 1:
1.6¡Á105.
1 EXPERIMENTAL
1.1 Apparatus and Reagents
MP-1 voltammetric analyser, produced by Shandong No. 7 Electric Communication Factory,
with three-electrode system composed of a dropping mercury electrode or a hanging mercury
drop electrode as working electrode, a platinum wire electrode as auxiliary electrode and
a saturated calomel electrode (SCE) as reference electrode. JM-01 hanging mercury drop
electrode, made by Jiangsu Electric Analysis Apparatus Factory. Incubation for the immune
reaction was carried out in a Model HH.W21.Cr420 incubator, produced by Guangdong Shantou
Instrument Factory.
o-Aminophenol (OAP): Fluka, 5.0¡Á10-3 mol/L OAP solution was
prepared by dissolving 0.0546 g OAP in 10 mL alcohol and diluted to 100 mL with water. HRP
solution : Dongfeng Biochemical Technique Co., Shanghai Institute of Biochemistry, 250
units per mg enzyme (RZ>3.0), the 1.0¡Á10-3 g/mL stock solution of HRP was
prepared by dissolving 0.0500 g HRP in 50 mL water, which was stored in a refrigerator at
4¡ãC. H2O2 solution: 4.0¡Á10-4 mol/L, prepared before
using. Britton-Robinson buffer solution: 0.2 mol/L, pH 8.0. Carbonate buffer (0.1 mol/L,
pH 9.6 ) was prepared by dissolving 1.60 g Na2CO3 and 2.90 g NaHCO3
in water and diluted to 1 L. pH 7.4 PBS-Tween 20 buffer solution was prepared by
dissolving 8.00 g NaCl, 2.90 g Na2HPO4.12H2O,
0.20 g KH2PO4, 0.20 g KCl and 0.5 mL 1% Tween 20 in water and
diluted to 1 L. Bovine serum albumin solution (1%) was prepared by dissolving 1 g bovine
serum albumin in 100 mL PBS buffer. The goat antirabbit IgG-HRP was purchased from Beijing
Biotinge Biomedicine Co.. Substrate solution: to a colorimetric tube of 10 mL were in turn
added 2.0 mL of 5.0¡Á10-3 mol/L OAP solution,1.0 mL of 4.0¡Á10-4
mol/L H2O2 solution, 0.5 mL of 0.2 mol/L BR buffer solution ( pH 8.0
). Then the mixture was diluted to the desired scale and shaken to uniform. The other
reagents were all analytical reagents prepared with doubly deionized water.
1.2 Antigen and antibody
The purified SBMV and the antibody of SBMV (SBMV-Ab) were prepared according to the
reference [9]. The infected leaf sap to be analyzed was obtained from the following
procedure[9]: 2.0 mL of 0.2 mol/L phosphate buffer ( pH 7.2 ) was added into
1.0 g fresh infected leave, ground and filtered. The filtrate was the infected leaf sap.
1.3 Measurement of HRP activity
To a colorimetric tube of 10 mL were in turn added 2.0 mL of 5.0¡Á10-3
mol/L OAP solution, 1.0 mL of 4.0¡Á10-4 mol/L H2O2
solution, 0.5 mL of 0.2 mol/L BR buffer solution ( pH 8.0 ), 1.0 mL of 1.0¡Á10-9
g/mL HRP solution; this was diluted to the desired scale and shaken to make them mixed
homogeneously. The mixture was set for 30 min at a water bath of 37¡ãC. The solution was
transferred into a cell of 10 mL. The second order derivative linear-sweep voltammogram
was recorded with a MP-1 voltammetric analyser. The working conditions were as follows:
the initial potential, -0.50 V; the potential scanning rate, 535 mV/s; and the mercury
drop standing time, 12.8 s.
1.4 Method for determination of SBMV
The purified SBMV and infected leaf sap were diluted with 0.1 mol/L pH 9.6 carbonate
buffer, respectively. The polystyrene immunoplate was coated with the diluted antigens,
200 mL per well; It was
incubated at 37¡ãC for 2 h and then placed in a refrigerator of 4¡ãC for 12 h. The well
was emptied and then 300 mL
PBS-Tween 20 buffer was added to per well; It was set for 3 min; The well was emptied.
This manipulation was repeated for three times. The plate was coated with 300 mL of 1% bovine serum albumin; It was
incubated at 37¡ãC for 30 min. The plate was washed as above. 200 mL of appropriate concentration of
SBMV-Ab ( 1:6400 ) was added to per well; It was incubated at 37¡ãC for 2 h. The plate was
washed again. 200 mL of
IgG-HRP ( 1:640 ) was added to per well; It was incubated at 37¡ãC for 2 h. The plate was
washed as above and washed for another time with doubly deionized water. 500 mL of substrate solution was added to
per well; It was set for 30 min at a water bath of 37¡ãC. The electrochemical probes were
inserted directly into the solution to record the second order derivative linear-sweep
voltammogram.
2 RESULTS AND DISCUSSION
2.1 Second order derivative linear-sweep voltammograms
The product of enzyme-catalyzed reaction has well-defined voltammetric peak. Figure 1
shows the results of the second order derivative linear-sweep voltammograms. Curve 1 is
the voltammogram of BR buffer solution, which has no voltammetric peak. Curve 2 is that of
BR + OAP + H2O2, which has a small voltammetric peak at -0.87 V.
Curve 3 is that of the enzyme-catalyzed reaction solution. Owing to the addition of HRP,
which accelerates greatly the oxidation of OAP with H2O2, the
reaction product produces a big and well-defined voltammetric peak at -0.87 V.
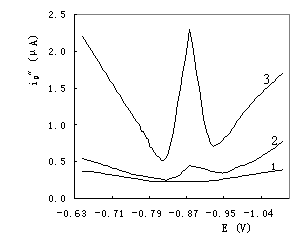 |
Fig.1 Second order
derivative linear-sweep voltammograms 1. 0.01 mol/L pH 8.0 BR buffer; 2. reaction without HRP: 0.01 mol/L pH 8.0 BR buffer + 1.0¡Á10-3 mol/L OAP + 4.0¡Á10-5 mol/L H2O2; 3. reaction with HRP: 2 + 1.0¡Á10-10 g/mL HRP. |
2.2 Conditions for the enzyme-catalyzed reaction
HRP catalyzes the oxidation reaction of OAP with H2O2 in BR
buffer solution. From the catalytical cycle of HRP in reaction[10], the
processes of the enzyme-catalyzed reaction can be expressed as follows:
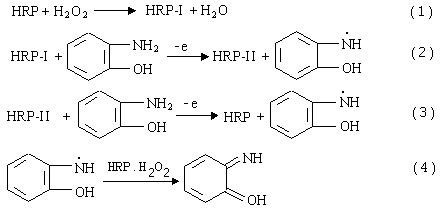
HRP-I and HRP-II are the intermediates of HRP.
In pH 7.8 - 8.2, a
sensitive and stable polarographic peak can be obtained. So BR buffer at pH 8.0 was chosen
as optimal enzymatic reaction solution. The concentrations of BR buffer solution, OAP and
H2O2 were also studied. When 10 mL of the overall reaction solution
includes 0.5 mL of 0.2 mol/L pH 8.0 BR buffer solution, 2.0 mL of 5.0¡Á10-3
mol/L OAP solution and 2.0 mL of 4.0¡Á10-4 mol/L H2O2 solution,
the peak is the highest and also stable.
Under the selected enzyme-catalyzed reaction conditions, the peak
height increases with the reaction time. The peak height achieved is high enough for
detection at 30 min at a water bath of 37¡ãC. And the reaction rate keeps stable within 60
min. 30 min was selected as the time for the enzyme-catalyzed reaction at a water bath of
37¡ãC.
2.3 The electrode processes of the product of the
enzyme-catalyzed reaction.
For pH 8.0 BR buffer solution, the linear-sweep voltammetric peak increases with the
increase of the rate of scanning. But the plot of the peak current against the square root
of the rate of scanning is not linear but an upward curve in the range of 186 mV/s - 1038
mV/s. The peak potential values shift to more negative values with the increase of the
rate of scanning. The electrocapillary curve of OAP-H2O2-HRP was
compared with those of OAP-H2O2 and BR buffer solution,
respectively. Between 0.00 V and -2.00 V, the surface tension of the former solution is
much less. The above results indicate that the product of the enzyme-catalyzed reaction is
adsorbed on the mercury electrode.
Under the selected conditions, the multiple-sweep cyclic voltammograms of the product of
the enzyme-catalyzed reaction were recorded. On the first scanning curve, there is a
cathodic peak but no anodic peak. Based on the theory advanced by Nicholson[11],
this is a two-electron irreversible electrode process. In the multiple-sweep cyclic
voltammograms, the cathodic peak gradually disappears with the increase of the scanning
number. This is the adsorptive behaviour of the product of the enzyme-catalyed reaction.
From the above experimental results, we can have a two-electron irreversible reduction
process of o-benzoquinone imine in pH 8.0 BR buffer solution, which can be expressed as
follows:
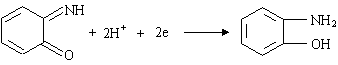
2.4 Determination of HRP
According to the experimental method, different quantities of HRP were used to
catalyze the oxidation reaction of OAP with H2O2 and the second
order derivative linear-sweep voltammograms were recorded. The HRP concentration from 6.0¡Á10-12
g/mL to 4.0¡Á10-9 g/mL has a good linear relation with the peak height in BR
buffer solution. The relative standard deviation is 2.9% for eleven parallel
determinations with 6.0¡Á10-12 g/mL HRP, and the detection limit of HRP is 3.5¡Á10-12
g/mL ( 3s ).
2.5 Determination of IgG-HRP
In order to use the new system in the immunoassay of different plant viruses, the
IgG-HRP was detected under the optimal experimental conditions. After the IgG-HRP was
adsorbed on the solid-phase carrier, the effect of the solid-phase carrier on the
determination was studied. As for the above mentioned IgG-HRP, the highest dilution ratios
detected by this method are 1: 8.0¡Á107; 1:1.0¡Á106. The highest dilution ratios detected by the
o-phenylenediamine spectrophotometric ELISA method are 1:1.6¡Á107;
1: 2.1¡Á105. The detection limits of this method are
lowered by 5 times, respectively, compared with those of the o-phenylenediamine
spectrophotometric ELISA method. The presented method is a high sensitive detection one.
2.6 Determination of SBMV
SBMV was detected with indirect method. Nonspecific adsorption of SBMV and labelled
antibody can be suppressed by using Tween 20 and BSA. The working concentrations of
SBMV-Ab and IgG-HRP were selected. The polystyrene immunoplate was coated with the
purified SBMV of 10mg/mL. The SBMV-Ab was multiply diluted with PBS-Tween 20. The
experiment was conducted according to the procedure with 1:640 IgG-HRP and SBMV-Ab of
different dilution ratios. The peak is the highest when the dilution ratio of SBMV-Ab is
1:6400. Similarly, the experiment was conducted with 1:6400 SBMV-Ab and IgG-HRP of
different dilution ratios. According to the principle that the labelled antibody
concentration should be at the upper point of the one third of the linear portion of the
concentration curve of the labelled antibody[9], the selected working
concentration of IgG-HRP is 1:640. Under the optimized conditions, the linear range for
the determination of the purified SBMV is 8.0 - 5000 ng/mL and the detection limit is 8.0
ng/mL. The equation of linear regression is ip" = -2.27 + 5.45 log2C
(n=8, r=0.9903). The relative standard derivation is 4.1% for eleven parallel
determinations with 60.0 ng/mL SBMV solution. When the o-phenylenediamine
spectrophotometric ELISA method is used, the linear range of the purified SBMV is 40.0 -
5000 ng/mL and the detection limit is 40.0 ng/mL. The equation of linear regression is A =
-0.521 + 0.464 logC ( n = 8, r = 0.9942). The detection limit of this method is 5 times
lower than that of the o-phenylenediamine spectrophotometric ELISA method.
2.7 Determination of SBMV-infected leaf sap
Under the optimized conditions, the infected leaf sap was analyzed. The results of
this method and o-phenylenediamine spectrophotometric ELISA method were listed in Table 1.
The highest dilution ratio detected with this method is 1:1.6¡Á105 and the
detection range is 1:1.6¡Á 105 - 1:2.5¡Á102. The highest dilution
ratio detected is 1:3.1¡Á104 and the detection range is 1:3.1¡Á104 -1:2.5¡Á102
with the o-phenylenediamine spectrop