http://www.chemistrymag.org/cji/2000/02c055ne.htm |
Dec. 2, 2000 Vol.2 No.12 P.55 Copyright |
The effect of water adsorption on calcined zeolite HZSM-5 by 1H MAS NMR spectroscopy
Chen Tiehong , Sun Pingchuan#, Sun
Shiwei, Han Wenhui, Wang Jingzhong, Ding Datong##
(Department of Chemistry, #Institute of Polymer, ##Department of
Physics, Nankai University, 300071)
Received Aug.10, 2000; Supported by the National Natural Science Foundation of China (Grant No. 29803004)
Abstract By 1H MAS NMR study,
it was found that the calcination did not directly cause dealumination in zeolite HZSM-5
if the sample is not exposed to water. During the calcination, some of the Al-O bonds of
the Bronsted acidic sites may be partially broken. When the calcined samples are exposed
in the moisture, the adsorbed water will interact with the partially broken bond and
dealumination takes place. A proton signal at 2.7ppm was found to be another kind of
adsorbed water besides the 6.5ppm adsorbed water, which should be differentiated between
the extra-framework aluminum hydroxyls.
Keywords Water, Zeolite HZSM-5, 1H MAS NMR
Zeolites have widely been used as catalysts in the cracking and isomerizaiton of alkanes. Due to the excess of negative charges generated by the incorporation of Al atoms in the zeolite framework, Bronsted acid sites are formed by the compensating bridging hydroxyl protons. It has been reported that by proper thermal treatment the catalytic activity of zeolites increased greatly, the key point may be the change of Al coordinations. Unfortunately, due to the strong quadrupolar constant of Al nuclei in zeolites at dehydrated state, it is very difficult to observe the 27Al NMR signal directly. The 1H NMR study on the protons in zeolites may provide structure information on Al coordinations and dealumination. In this note the 1H MAS NMR results show that the hydration will be an important factor to the Al coordination change in the calcined zeolite HZSM-5.
1 EXPERIMENTAL
Zeolite Na-ZSM-5(Si/Al ratio=13) was repeatedly ion-exchanged by 0.2N HNO3
solution and washed by de-ioned water to get the parent HZSM-5 sample. All the treatment
of samples are listed in the following table.
Samples ID |
Treatment |
C600(650,700) |
HZSM-5, calcined at 600(650,700¡ãC) under 10-3 torr for 3 hrs and sealed for NMR measurments (without contact of moisture) |
C600(650,700)H |
Samples C600(650,700), were put in a desiccator with saturated NH4Cl solution for 5 days for full hydration, and were evacuated at 10-3 torr, 400¡ã C for 5hrs, then sealed for NMR measurments. |
1
H MAS NMR spectra were measured on Varian UNITY plus 400 NMR spectrometer at 400.005MHz, with 8 s recycle time, 4.5 kHz spin rate, and 32 accumulations. The sealed tubes were opened, and the samples were packed into NMR rotor in a glovebox filled with high-purity N2. The rotors with samples were measureed again after 5 hrs and the results prove that the rotor is well moisture-proof.2 RESULTS AND DISCUSSION
The 1H MAS NMR spectra of C600(650,700)H are similar with those reported in the
literatures [1,2], and several signals can be distinguished, i.e., 2.0ppm
silanol groups, 4.2ppm Bronstd acid sites (its intensity gradually became smaller due to
the dealumination process if the calcination temperature is higher), the 6.5ppm which has
been proved to be another kind of Bronsted acid sites [1], and the 2.7ppm
signal representing the extra-framework Al hydroxyls. There also appeared the 1.0ppm
signal which could be assigned to the hydroxyls at the surface defect.
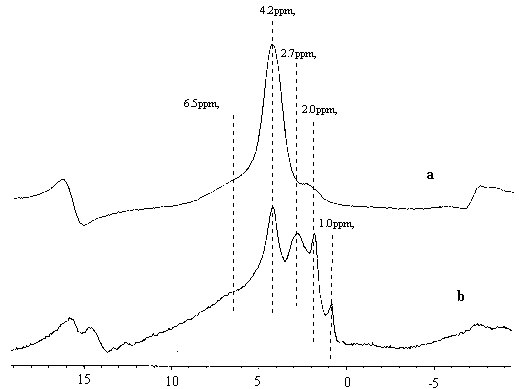
Fig 1 1H NMR spectra of (a)C700 (b)C700H
Compared with those of the C600(650,700)H
samples, the spectra of the C600(650,700) samples were quite different, i.e., the 4.2 ppm
signal of B acid sites remains almost unchanged, dominating the whole spectrum. In fig 1,
the 1H NMR spectra of C700 and C700H were compared. The two spectra are quite
different. In Fig 1a, just after calcination at 700¡ãC(without contact of moisture), the
4.2 ppm B acid sites dominated the whole spectrum. The 6.5ppm signal can also be
distinguished as another kind of Bronsted acidic sites. The signal of silanol groups at
around 2.0ppm displayed a rather broad pattern, indicating that its complexity just after
the calcination. It should be noted that after hydration(contact of water), the 4.2ppm
signal greatly decreased and 2.7 ppm extra-framework hydroxyls, as well as the 2.0ppm
sharp line(silanol groups) appeared,. It is clearly seen that the hydration at room
temperature caused irreversible change of the framework, i.e., after calcination the
hydration process directly results in dehydroxylation and dealumnation.
Besides the 6.5ppm bridging hydroxyls [1], the 6.5ppm signal
were also assigned to the adsorbed water on Lewis acid sites [2,3]. In our
experiments, as displayed in Fig 2, there is some residual adsorbed water at 6.5ppm if the
outgas temperature was below 400¡ãC. Meanwhile, it should be
noted that there is another signal at 2.7ppm in the spectra of 150 and 250¡ãC outgassed
samples. Hydroxyls at this position normally is assinged to be the extra-framework Al
hydroxyls [5], but in our parent sample, there is no obvious dealumination so
there should not be some extra-framework Al hydroxyls, and it can be clearly seen that in
the spectrum of the 400¡ãC outgassed sample this 2.7ppm signal
disappeared. In contrast, as shown in Fig 3, in the 650¡ãC
calcined and hydrated sample, after 400¡ãC outgas, the 2.7ppm
remain still as the extra-framework Al hydroxyls. Also in Fig 3, the 2.7ppm signal became
more obvious in the 150 and 250¡ãC outgassed samples. So we
assigned the newly found 2.7ppm signal as another kind of water adsorbed signal.
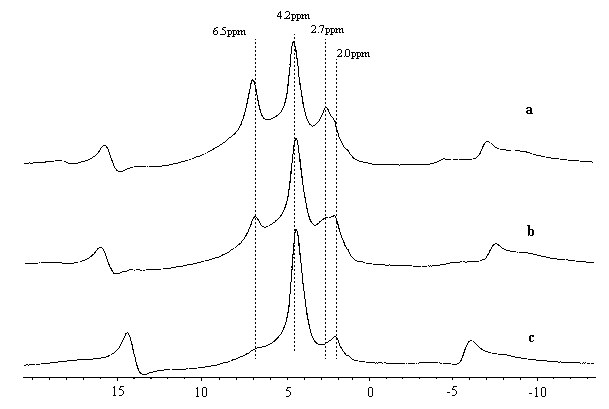
Fig 2 1H NMR of HZSM-5 evacuated
at (a)150¡ãC(b) 250¡ãC (c) 400¡ãC
3 CONCLUSION
By 1H MAS NMR study, it was found that the calcination did not directly cause
dealumination in zeolite HZSM-5 if the sample is not exposed to water. During the
calcination, some of the Al-O bonds of the Bronsted acidic sites may be partially broken.
When the calcined samples are exposed to moisture, the adsorbed water will interact with
the partially broken bond and dealumination takes place. A proton signal at 2.7ppm was
found to be another kind of adsorbed water besides the 6.5ppm adsorbed water, which should
be differentiated between the extra-framework aluminum hydroxyls.
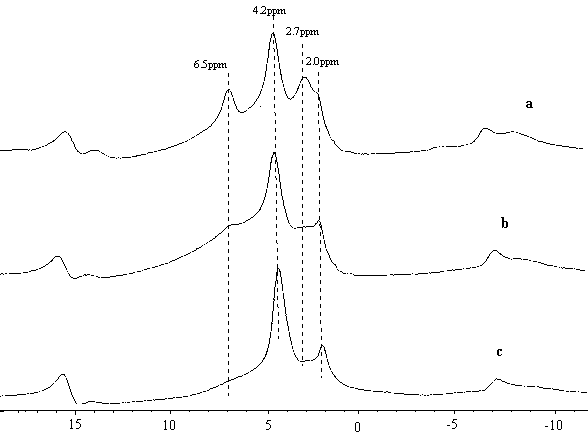
Fig 3 1H NMR of HZSM-5 calcined at 650¡ãC, hydrated and evacuated at
(a)150¡ãC (b) 250¡ãC (c) 400¡ãC
REFERENCES
[1] Beck L, White J, Haw J. J. Am. Chem. Soc., 1994, 116: 9657.
[2] Deng F, Du Y, Ye J. J. Phys. Chem., 1995, 99: 15208.
[3] Freude D. Chem. Phys. Lett., 1995, 235: 69.
[4] Hunger M, Freude D, Pfeifer H. J. Chem. Soc., Faraday Trans., 1991, 87: 657.
[5] Freude D, Ernst H, Wolf I. Solid State NMR, 1994, 3: 271.