http://www.chemistrymag.org/cji/2003/053021pe.htm |
Mar. 1, 2003 Vol.5 No.3 P.21 Copyright |
Kinetics and mechanism of oxidation of b -propylene-glycol by diperiodatonickelate (IV) ion in alkaline medium
Shan Jinhuan, Qian Jing, Wang Liping, Shen Shigang, Sun Hanwen( College of Chemistry and Environmental Science, Hebei University, Baoding 071002,China)
Received on Nov.25, 2002; Supported by the Natural Science Foundation of Hebei Province ( No.295066)
Abstract The kinetics of oxidation of b-propylene-glycol (b-PG) by dihydroxydipriodatonickelate ( IV) ion ( DPN) was studied by spectrophotometry in alkaline medium. The reaction was found to be pseudo-first order with respect to Ni( IV) and to be fractional order with respect to
b-PG. The rate increased with the increase of the concentration of OH- and decreased with the increase of the concentration of IO4-. Added salts did not affect the rate and no free radical was detected. A mechanism involving a pre-equilibrium of an adduct formation between the complex and b-PG was proposed. The rate equation derived from the mechanism can explain all the experimental results. The activation parameters of the rate-determining step were calculated.Keywords dihydroxydipriodatonickelate (IV), b-propylene-glycol, redox reaction, kinetics and mechanism
In recent years, study of the highest
oxidation state of transition metals intrigued many researchers' interests, which can
provide new and valuable information in some fields.
Transition metals in a higher oxidation state can generally be
stabilized by chelation with suitable polydentate ligands. Metal chelates such as
diperiodatocuprate ( III) [1], diperiodatoargentate( III) [2],
diperiodatonickelate( IV) [3] and nickel ( IV) oxime imine[4]
complexes are good oxidants in a medium with an appropriate pH value. The use of Ni( IV)
as an oxidizing agent is well known in the investigation of some organic compounds such as
tetrahydrofurfuryl alcohol[5], 1,4-dioxane[6], ethylene diamine[7]
etc. In the previous works, the existent form was [Ni(OH)2 (H3IO6
1 EXPERIMENTAL
1.1 Materials
All reagents used were of A.R. grade. All solutions were prepared with twice-distilled
water. Solutions of DPN and
1.2 Kinetics measurement and reaction product analysis
Measurements of the kinetics were performed using a UV-8500 spectrophotometer (Shanghai) fitted with a 501 thermostat ( ¡À0.1ºC) . Details of the determinations are described elsewhere [10]. The oxidation product was identified as the corresponding aldehyde by spot test[11]. 2 RESULTS AND DISCUSSION
2.1 Evaluation of pseudo-first order rate constants
Under the conditions of [b-PG]0 >> [Ni( IV) ]0, the plots of ln(At-A¡Þ) vs. time t for more than three times the half-life of the reaction were good straight lines ( r>0.9997) , indicating the order in Ni( IV) to be unity. The pseudo-first-order rate constants kobs were calculated by using the least-squares method. The rate constants reported here are average of three independent runs. Deviations in duplicate determinations are generally less than ¡À 5% .
2.2 Effect of varying [b-PG]
Under the condition of [b-PG]0 > > [ DPN]0, at fixed [Ni(IV) ], [OH-], [IO4-], ionic strength I and temperature, kobs values increased with the increase in concentration of b-PG and the order with respect to b-PG was found to be fractional ( in the temperature range of 25- 45ºC, nap= 0.44- 0.68) . The plots of 1/ kobs vs. 1/ [b-PG] were straight lines with a positive intercept ( r>0.996) ( Fig.1) .
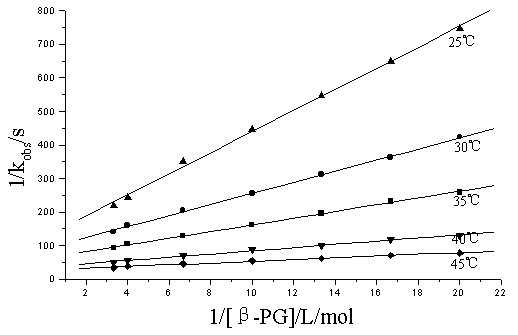
Fig.1 The plots of 1/ kobs vs. 1/ [b-PG] at different temperatures
[Ni( IV) ]= 0.641¡Á10-4mol/ L; [IO4-]= 1.195¡Á10-3mol/ L;
[OH-]= 1.498¡Á10-2mol/ L; I= 1.62¡Á10-2mol/ L
2.3 Effect of varying [OH-]
At constant [Ni( IV) ], [
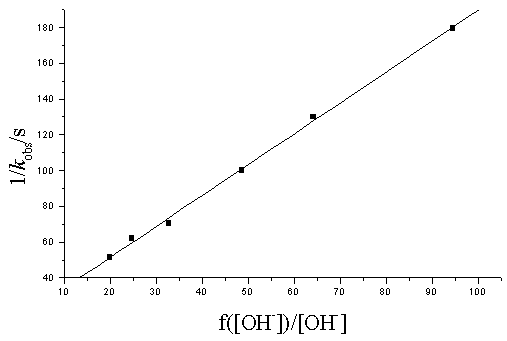
Fig.2 The plot of 1/ kobs vs f( [ OH-] / [ OH-] ) at t= 35ºC
[ Ni( IV) ] = 0.641¡Á10-4mol/ L;[ IO4-] = 1.695¡Á10-3mol/ L; [b-PG] = 0.2mol/ L; I= 4.88¡Á 10-2mol/ L
2.4 Effect of varying [IO4-]
At fixed [Ni( IV) ], [b-PG], [OH
2.5 Effect of varying I
At fixed conditions except ionic strength I, kobs hardly altered with the increase in I ( Table 1) .
Table 1 Influence of Variation of I, and [IO4-]
104[Ni( IV) ]/ mol/ L |
102[OH-]/ mol/ L |
103[IO4-]/ mol/ L |
102I/ mol/ L |
103kobs / s-1 |
0.458 |
0.712 |
0.711 |
1.05 |
8.94 |
0.458 |
0.712 |
1.211 |
1.05 |
6.38 |
0.458 |
0.712 |
1.711 |
1.05 |
5.08 |
0.458 |
0.712 |
2.411 |
1.05 |
3.79 |
0.458 |
0.712 |
3.411 |
1.05 |
2.75 |
0.641 |
1.498 |
1.495 |
1.647 |
9.69 |
0.641 |
1.498 |
1.495 |
2.647 |
9.91 |
0.641 |
1.498 |
1.495 |
3.647 |
10.10 |
0.641 |
1.498 |
1.495 |
5.147 |
11.02 |
0.641 |
1.498 |
1.495 |
6.647 |
10.80 |
[
b-PG]= 0.2mol/ L ; t= 35ºC2.6 Discussion
In the alkaline medium, the dissociative equilibria ( 1) - ( 3) of the IO4-
were detected and the corresponding equilibrium constants at 25ºC were determined by
Aveston[12].
2IO4- + 2OH-![]() H2I2O104-
logb1 = 15.05
( 1)
H2I2O104-
logb1 = 15.05
( 1)
IO4- + OH- + H2O ![]() H3IO62-
log
H3IO62-
log
IO4- + 2OH-
The distribution of all periodate species in alkaline solution was calculated from equilibria ( 1) - ( 3) . The dimer ( H2I2O104-) and IO4- species of periodate can be neglected. The main species of periodate are H3IO62- and H2IO63-, consistent with the result calculated from Crouthamel's data[13] by Murthy. Based on such distribution, the formula of Ni( IV) periodate complex may be represented by either [Ni(OH)2(H3IO6) 2]2- or the less protonated [Ni(OH)2 (H2IO6)2]4-. We preferred to use the latter to represent DPN because it is close to that suggest by Mukherjee[14] and will obtain support from the kinetic studies.
In view of the above results and discussion, a plausible reaction mechanism was proposed:
[Ni(OH)2(H2IO6)2]4- + OH-
I II
[Ni(OH)2(HIO6)]2- + CH2OHCH2CH2OH
II
complex
Here, reaction ( 6) was the rate-determining step.
Subscripts T and e stand for total concentration and concentration at equilibrium respectively.
As the rate of the disappearance of Ni( IV) was monitored, the rate of the reaction can be derived as:
Neglecting the concentration of ligand dissociated from Ni( IV) and the species of periodate other than H2IO63- and H3IO62-, here:
[IO4-]ex @ [H3IO62-]+ [H2IO63-] ( 9)
Equations ( 10) and ( 11) can be obtained from ( 2) , ( 3) , ( 9) :
Substituting eq ( 10) into ( 8) , we can get the following equations:
Eq ( 12) suggests that the plot of 1/ kobs vs 1/ [b-PG] should be line, and eq ( 13) shows that the plot of 1/ kobs vs f ( [OH-]) / [OH-] should be line, eq ( 14) shows that the plot of 1/ kobs vs [IO4-]should also be line, which are consistent with the experimental results.
If the formula of DPN was [Ni(OH)2 (H3IO6)2]2-, getting eq ( 11) into ( 8) obtained eq ( 15) :
The plot of 1/ kobs vs f ( [OH-]) / [OH-] should also be line, but the linearity was not straight ( Fig.3) , which substantially denies eq( 15) . Therefore, it seems advisable to represent DPN by [Ni(OH)2(H2IO6)2]4-, which is consisted with the experimental observation.
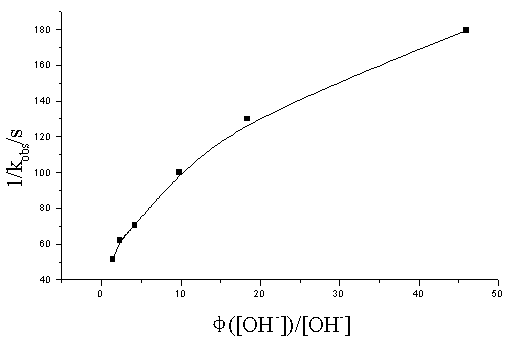
Fig.3 The plot of 1/ kobs vs. f( [OH-]) / [OH-] at t=35ºC
[Ni( IV) ]= 0.641¡Á10-4mol/ L; [IO4-] = 1.695¡Á10-3 mol/ L; [b-PG] = 0.2 mol/ L; I = 4.88¡Á10-2 mol/ L
Activation energy and the thermodynamic parameters ( t= 25
ºC) were evaluated ( Table 2) by the method given earlier[15].Table 2 Rate Constants of Rate-Determining Step and Activation Parameters
T / K |
298.2 |
303.2 |
308.2 |
313.2 |
318.2 |
activation parameters( 25 ºC) |
|
|
|
|
|
|
Ea=61.61kJ/ mol DS¡Ù= -87.30J/ K·mol |
r= 0.995,a = 19.96, b = - 7408.96 for the line regression of lnk vs 1/ T.
REFERENCES[ 1] Niu W J, Zhu Y, Hu K C et al. Int. J. Chem. Kinet, 1996, 28 (12) : 899.
[ 2] Shi T S. Science in China ( series B) ( Zhongguokexue B) , 1990, 33: 471.
[ 3] Chandraiah U, Murthy C P, Sushama Kandlikar. Indian J. Chem. 1989, 28A: 248.
[ 4] Santanu B, Pradyot B. Bull. Chem Soc. Jpn., 1996, 69: 3475.
[ 5] Li Z T, Wang F L, Wang A Z. Int. J. Chem. Kinet, 1992, 24: 933.
[ 6] Halligudi N N, Desai S M, Nandibewoor S T. Int. J. Chem. Kinet, 1999, 31: 789.
[ 7] Li Z T, Chang Q, Li B W, et al. Chinese Research in Chinese Universities (GaodengxuexiaoHuaxuexuebao) , 2000, 21 (5) : 747.
[ 8] Baker L C W, Mukherjee H G, Sarkar S B et al., Indian J. Chem., 1982, 21A: 618.
[ 9] Murthy C P, Sethuram B, Navaneeth Rao T. Z. Phys. Chemie, Leipzig, 1986, 287: 1212.
[ 10] Shan J H, Liu T Y. Acta Chimica Sinica (Huaxue xuebao) , 1994, 52: 1140.
[ 11] Feigl F. Spot Tests in Organic Analysis, New York: Elsevier Publishing Co., 1966, 7: 196.
[ 12] Aveston J. J. Chem.Soc. ( A) , 1969: 273.
[ 13] Crouthamel C E, Meek H V, Martin D S et al. J. Am. Chem. Soc., 1949, 71: 3031.
[ 14] Mukherjee H G, Mandal B, et al. Indian J. Chem., 1984, 23A: 426.
[ 15] Shan J H, Wang L P et al. Chinese Journal of Inorganic Chemistry (Wujihuaxuexuebao), 2002, 18 ( 9) : 887.
[ 16] JIN J J. Kinetics Principle of Chemical Reaction in Liquid Phase., Shanghai: Science
Technique Press, 1984: 29.
[ 17] The Teaching and Research Section of Analytical Chemistry in Zhongnan Mining Institute, Handbook of Analytical Chemistry, Beijing: Science Press Co., 1984: 567. ¡¡
¡¡