http://www.chemistrymag.org/cji/2004/062015pe.htm |
Feb.21, 2004 Vol.6 No.2 P.15 Copyright |
(Key laboratory of analytical science and technology of Hebei province, College of Chemistry and Environmental Science, Hebei University, Baoding, 071002, China)
Received Jan. 15, 2004; Supported by the Natural Science Foundation of Hebei province, China.(No.203110)
Abstract A reversed-phase ion-pair
chromatography method with ammonium tetrabutyl bromide as ion-pairing reagent and UV
detection has been developed for aromatic sulfonated compounds. It was testified that the
concentration of ion pair reagent, pH and the volume ratio of tetramethylene-oxide in
mobile phase played important roles in the separation. Linearity regression equations
along with linearity ranges, correlation coefficients of 0.9991-0.9996 and detection limit
for determination of seven sulfonated compounds were offered. To achieve the detection of
analytes in low concentration water samples, the ion-pair extraction technique was
employed with ammonium tetrabutyl bromide (TBA) as ion-pair reagent and dichloromethane as
extractant. Recoveries of the method were between 96.8 and 101.7%, and relative standard
deviation was below 7.6%. Different concentration of 3-nitro-benzenesulfonate,
dibenzal-4-sulfonate and methyl orange were detected from rainwater, tap water and
electrodialysised water sample, respectively.
Keywords Reversed-phase ion-pair chromatography, sulfonates, tetramethylene-oxide,
ion-pair extraction
1. INTRODUCTION
Aromatic sulfonates such as benzene sulfonates and naphthalenesulfonates are widely
used as intermediates in the chemical industry, particularly in the production of
pharmaceuticals, dyes, tanning agents, pesticides, cement, concrete and so on. Due to
their excellent water-solubility and hydrophilic properties, it is difficult to remove
them completely from waste water in the water treatment works and they are discharged into
the aquatic environment[1]. The toxicology and environmental ecology research
is just underway[2,3]. Low biodegradability makes them potentially hazardous[4].
Various analytical techniques have been proposed for determining these compounds in water.
Gas chromatography analysis needs a previous step to convert them into volatile derivates[5].
When capillary electrophoresis is used, good results have been obtained particularly using
laser induced fluorescence detection[6]. The preferred technique, however, is
still ion-pair liquid chromatography with UV [7], fluorescence[8] or
MS[9].
The detection limits obtained with these techniques are not low enough
for them to be determined in real water samples. For this reason, an enrichment step is
necessary. Ion-pair extract technique is a method to improve the solubility of ionic
compounds in organic solvent through the formation of neutral associated compounds with
ion-pair reagent[10]. To improve the detection sensitivity and veracity of
sulfonates, it is necessary to establish the fast analysis method[11]. The aim
of this paper is to develop a method for determining a mixture of benzene sulfonates, and
naphthalenesulfonates. The method is based on ion-pair liquid chromatography with UV
detection, coupled with ion-pair extraction. To our knowledge, the simultaneous detection
of mixed benzene and naphthalene sulfonate compounds (4-aminobenzenesulfonate, benzenesulfonate, 2-naphthol-6,8-disulfonate,
2- naphthol-3,6- disulfonate, 3-nitro-benzenesulfonate, dibenzal-4-sulfonate, methyl orange) in
water samples has scarcely been studied.
2. EXPERIMENTAL
2.1 Instrument
The sensitive UV absorption wavelengths were taken by a Tianmei 8500 spectrophotometer
(Shanghai, China). The pH were detected with a Tianmei PHS-3C acidometer (Shanghai,
China).
The chromatographic system consisted of the Shimadzu corporation
commercial components: a solvent-delivery pump, a SPD-6AV UV-VIS detector, operating at
220 nm. The data were collected and analyzed by using CR-3A system (Kyoto, Japan).
Separations were performed on a C18 column (25.0 cm¡Á0.46 cm 5
2.2 Reagents and the Preparation of Samples
Ammonium tetrabutyl bromide(analytical grade), tetramethylene-oxide (analytical grade, fresh distillated before use), methanol (HPLC quality), and the following sulfonated compounds were analytical reagent and purchased from Beijing: 4-aminobenzenesulfonate, benzenesulfonate, 2-naphthol-6,8-disulfonate, 2- naphthol-3,6- disulfonate,3-nitro-benzenesulfonate, dibenzal-4-sulfonate, methyl orange. All the other chemicals were commercial analytical reagent. Double-distilled water was used for preparing the mobile phase.
For the experiments, a stored water solution of 1g L-1 of each compound is prepared. Standard solutions of each compound in the concentration of 0.001-0.1g L-1 are prepared with the mobile phase as diluent, respectively.
Samples were collected with 500 mL pre-cleaned amber glass bottles, filtered through a 0.45mm membrane filter and kept at 4oC until analysis. A 20 mL of the sample was enriched in a plugged-glass tube through the addition of a definite mass of extractant and ion-pair reagent under a definite pH value, and the extracts were analyzed with the mentioned chromatographic system.
3 RESULTS AND DISSCUSSION
3.1 The selection of ion pair reagent and its concentration
High performance liquid chromatography was not suited for either efficient retention or separation of the sulfonates. The addition of ion-pairing agents at appropriate concentration made them have efficient retention. Ammonium tetrabutyl bromide (TBA) had relatively low molecule mass than other ion-pair reagent, which was more beneficial to improve the separation selectivity of components with similar structure. The effect of TBA concentration on the retention of seven analytes was examined. The results were showed in Figure 1 and it illustrated that the sulfonated compound had a shortened retention time with the increasing of TBA content, and different component had different efficient column capacity.

Fig.1 The influence of TBA
concentration on sulfonates' retention behavior
(The mobile phase was methanol- water (30:70, v/v)- pH 6.5 and 5%THF for compound 1-5, 16%
THF for compound 6-7)
3.2 Influence of pH on retention behavior
To insure the formation of ion-pair, the pH value of mobile phase was adjusted ranging
from 3.5 to 7.5 by adding phosphoric acid or sodium hydrogen phosphate[12]. The
raising pH value resulted in the reduction of retention time as showed in Figure 2. That
might be caused by the increasing negative charge of phosphate, which promoted the
complexing of phosphate with TBA, then reduced the complexing of TBA with sulfonate
negative ion[13]. The affection of pH value on benzenesulfonate was very
slight, but the change of methyl orange was more notable. Through the adjustment of pH
value, good separation of sulfonates could be obtained.

Fig. 2 The influence of pH
value on sulfonates' relative retention factor
(The mobile phase was methanol- water (30:70, v/v)- TBA (3mmol/L) and 5 % THF for compound
1-5, 16 % THF for compound 6-7)
(1) 4-aminobenzenesulfonate (2) benzenesulfonate (3) 2-naphthol-6,8-disulfonate
(4) 2- naphthol-3,6-
disulfonate (5) 3-nitro-benzenesulfonate
(6) dibenzal-4-sulfonate
(7) methyl orange

Fig. 3 The influence of THF concentration on
sulfonates
((The mobile phase was methanol- water (30:70, v/v)- TBA (3mmol/L), pH 6.5)
(1) 4-aminobenzenesulfonate (2) benzenesulfonate (3) 2-naphthol -6,8-disulfonate (4) 2- naphthol-3,6- disulfonate (5) 3-nitro-benzenesulfonate (6) dibenzal-4-sulfonate (7) methyl orange
3.3 The influence of organic reagents on
retention
Aromatic sulfonates all possessed the
hydrophilic group and lipophilic group, which demanded the eluent should contain both
water and organic solvent. When the methanol content was 30%, the retention time of
3-nitro-benzenesulfonate was about 30min, and dibenzal-4-sulfonate, methyl orange was
larger than 1h, respectively. With the increasing of methanol concentration, the retention
times were shortened, but the separation of sulfonates was destroyed. THF was a more
efficient solvent than methanol in reducing the retention of components[14],
and it possessed low viscosity and UV absorption wavelength. In the experiment, the
retention behavior of sulfonates in 30:70 methanol-water (v/v) mobile phase containing
different content of THF was studied. According to the experimental results (showed in
Figure 3) we knew that THF could effectively reduce the retention
time of sulfonates. This was caused by the reduction of polarity of the mobile phase and
abasing the column capacity.
3.4 The Separation of Sulfonates
In the separation of sulfonates, the concentration of THF, methanol, TBA and the pH value
of the mobile phase was important influencing factor, and the content of THF was the
crucial factor in speedy separation. The mobile phase was methanol- water (30:70, v/v)-THF
(5%)-TBA (3 mmol/L), pH 6.5. Under isocratic flow rate of 1.0 mLmin-1, the
chromatograms were recorded as Figure 4, and the retention time of 4-aminobenzenesulfonate,
benzenesulfonate, 2-naphthol-6,8-disulfonate,2- naphthol-3,6- disulfonate and 3-nitro-benzenesulfonate was 3.14min, 4.33min,
4.99min, 5.30min and 7.45min, respectively. The analysis was much faster than that of
Gimeno[15], who used a mobile phase of methanol and water under different
gradients, with the retention time of 4-aminobenzenesulfonate, benzenesulfonate and 3-nitro- benzen esulfonate was 10min, 38min
and 52min, respectively. When the mobile phase was changed into methanol-water(30:70,
v/v)-THF(16%)-TBA (3 mmol/L), the retention time of dibenzal-4-sulfonate and methyl orange
was 6.04min and 9.17min (Figure 5), respectively.

Fig. 4 The chromatograms of
(The mobile phase was methanol- water (30:70, v/v)-THF (5%)-TBA (3 mmol/L), pH 6.5.)
tR,1= 3.14min, tR,2= 4.33min, tR,3= 4.99min, tR,4= 5.30min, tR,5= 7.45min
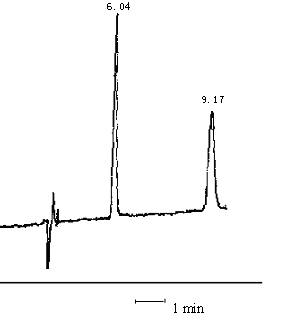
Fig. 5 The chromatograms of (1) dibenzal-4-sulfonate and (2) methyl orange
(The mobile phase was methanol- water (30:70, v/v)-THF (16%)-TBA (3 mmol/L), pH 6.5.)
3.5 Linearity and Detection Limit
The relation between peak area and mass of the component was examined. The linearity
regression equations along with linearity ranges, correlation coefficients and detection
limit for determination of the seven components were given in Table 1.
Table 1 The linearity and detection limit
Components |
Regression equation |
Linearity (mg) |
Correlation coefficient |
Detection limit(ng) |
4-aminobenzenesulfonate |
A=-372.4+50536.7X |
0.03-6.0 |
0.9992 |
3.9 |
benzenesulfonate |
A=751.6+14078.9X |
0.015-2.5 |
0.9994 |
8. 8 |
2-naphthol-6,8-disulfonate |
A=-236.7+43146.7X |
0.035-8.0 |
0.9991 |
4.1 |
2-naphthol-3,6-disulfonate |
A=514.6+30292.4X |
0.03-5.0 |
0.9995 |
3.3 |
3-nitro-benzenesulfonate |
A=1380.8+17128.5X |
0.025-8.0 |
0.9996 |
3.2 |
dibenzal-4-sulfonate |
A=554.6+8339.7X |
0.03-6.0 |
0.9994 |
2.3 |
methyl orange |
A=-249.3+71253.9X |
0.02-4.0 |
0.9996 |
3.9 |
A: the peak area, X: the mass of the components(
mg).3.6 The enrichment of samples
To achieve the determination of sulfonates existed in water samples, ion-pair extraction
was carried out with TBA as ion-pair reagent and dichloromethane as extractant. The
enriching condition was optimized by determination the influence of TBA concentration,
volume ratio of extractant to sample and pH value. The effect of TBA on extraction
recovery was more important than pH. When TBA equaled to 1.4 mg mL-1, the
maximum recovery of single extraction was obtained (showed in Figure 6). Table 2 showed
the results under the optimum condition of TBA (1.4 mg mL-1), dichloromethane-sample (1:20,v/v) and pH 6.0-7.0.
Recoveries of the method were between 96.8 and 101.7% (the data listed in the Table 2. had
been corrected with compound's extraction recovery), and relative standard deviation was
below 7.6%.

Fig. 6 The influence of TBA concentration on the extraction recovery
(The extraction was carried out under the condition of dichloromethane-sample (1:20,v/v) and pH 6.5)
(1) 4-aminobenzenesulfonate (2) benzenesulfonate (3) -2-naphthol-6,8-disulfonate (4) 2- naphthol-3,6- disulfonate (5) 3-nitro-benzenesulfonate (6) dibenzal-4-sulfonate (7) methyl orange
Table 2 Recoveries of the component
Components |
4-amino benzene sulfonate |
benzene sulfonate |
2-naphthol-6,8- disulfonate |
2-naphthol-3,6-disulfonate |
3-nitro- |
dibenzal-4-sulfonate |
methyl orange |
Recoveries (%) |
98.5 |
98.8 |
98.2 |
97.6 |
101.7 |
96.8 |
99.8 |
RSD% |
4.96 |
4.96 |
3.4 |
6.9 |
2.5 |
5.7 |
7.6 |
 |
 |
| Fig. 7
3-Nitro-benzene-sulfonate detected from rain water (The mobile phase was the same as that of Fig. 4) |
Fig. 8
Dibenzal-4-sulfonate and methyl orange detected from rain water |
Table 3 The detected sulfonates in samples
Sample |
The content of the components£¨ mg/L£© |
||
3-nitro-benzene-sulfonate |
dibenzal-4-sulfonate |
methyl orange |
|
Rainwater |
23 |
71 |
30 |
Tap water |
68 |
18 |
35 |
Electrodialyzed water |
13 |
7.6 |
2.4 |
4 CONCLUSION
In the fast analysis of dissociable compounds based on the reversed-phase ion-pair
chromatography, THF was an effective organic modifier for shortening analyzing time.
Ion-pair extraction of water sample made it possible for the detection of low
concentration sulfonates. The proposed method was simple and easy with good accuracy and
precision. It provided an effective detection measurement for the environmental
evaluation. If the analysis was performed by way of gradient elution, the simultaneous and
fast analysis of the seven compounds can be performed.
REFERENCE
[1] Alterbach B, Giger W. Anal. Chem, 1995, 67: 2325.
[2] Versteeg D J, Rawlings J M. Archives of Environmental Contamination and Toxicology,
2003, 44 (2): 237.
[3] Bruce E R, Patarapol T, Kuan C L et al. Biodegradation, 2001, 12 (1): 31.
[4] Ruff J, Hitzler T, Rein U et al. Appl Microbiol Biotechnol, 1999, 52 : 446.
[5] Reemtsma T J. Chromatogr. A, 1996, 733: 473.
[6] Cugat M J, Borrull F, Calull M. Chromatographia, 1999, 50: 229.
[7] Socher G, Nussbaum R, Rissler K, Lankmayr E. Chromatographia, 2001, 54 (1/2) : 65.
[8] Alonso M C, Castillo M, Barcelo D. Anal. Chem. Avta, 1999, 400:211.
[9] Storm T, Reemtsma T, Jekel M. J. Chromatogr. A, 1999, 854: 175.
[10] Li H B, Chen F, Xu X X. Fresenius J Anal. Chem, 2000, 367: 499.
[11] Poudrier J K. Today's Chemist at Work, 1996, 5 (4): 39.
[12] Chang W B, Li K A. Concise Handbook of Analytical Chemistry, Beijing: Beijing
university publishing house, 1981: 94.
[13] Pang X Y, Sun H W, Wang Y H. Chromatographia, 2003, 57 (7/8) : 543.
[14] Pang X Y, Sun H W, Shen S G. Chemical Journal on Internet, 2003, 5 (1) : 2.
[15] Gimeno R A, Marce R M, Borrull F. Chromatographia, 2001, 53 (1/2) : 22.