http://www.chemistrymag.org/cji/2004/068057pe.htm |
Aug. 18,
2004 Vol.6 No.8 P.57 Copyright |
Cai
Jiye, Chen Yao, Li Renqiang#, Xia Ke
(1 Department of Chemistry, Jinan
University, Guangzhou 510632, China; #Department of Biotechnology, Jinan University, Guangzhou
510632, China)
Supported in part by Grants-in Aid for Scientific Research from Guangdong Province, China (990483), the Ministry of Education, China (00124), and the National Natural Science Foundation of China (60278014 and 30230350).
Abstract Crystallization of calcium
oxalate with the self-assembly of organic matrix chondroitin sulfate film and aggregation
of these crystals on the film were studied using atomic force microscope. Results showed
that the formation and growth of calcium oxalate crystal were induced or controlled by
chondroitin sulfate molecule, and further these crystals aggregated to form Liesegang loop
on the film, which were due to the interaction between calcium oxalate and chondroitin
sulfate, especially to the reaction of calcium ion with those functional groups. The
formation of Liesegang loop of calcium oxalate was related to the concentrations of
calcium oxalate and chondroitin sulfate. These results would be in favor of the
understanding to the mechanism of mineral crystal growth controlled by organic matrix and
to the formation process of urinary calculus in human body due to that the calcium oxalate
is the main component of urinary calculus and the chondroitin sulfate is an important
component in human urine.
Keywords Calcium oxalate, chondroitin sulfate, atomic force microscope,
crystallization, Liesegang loop.
1. INTRDUCTION
Studies on the formation and growth of mineral constituent crystal in mimic
organic matrix system help us to understand the mechanism of calculus in human body. Some
studies on this field, especially on the formation of mineral crystal induced by LB film,
and on the Liesegang loop formed by these mineral crystal have been performed [1-3].
However, those organic substances existed in human body and their self-assembly process
may be related to the crystallization and aggregation of mineral crystal. Therefore, it is
helpful to use those organic substances existed in human body as the matrix to probe the
formation and growth of mineral crystal.
Urinary calculus is a normal disease happened in human. There are
mineral and organic constituents in the calculus, of which calcium oxalate is the main
mineral component. It is still a puzzle about the mechanism to form the urinary calculus,
so that in this study, the crystallization of calcium oxalate accompanying the
self-assembly of chondroitin sulfate molecules and its aggregation induced by chondroitin
sulfate film are imaged with atomic force microscope (AFM) due to chondroitin sulfate is
an important organic component in human urine, which is not yet presented in the previous
literatures. This study is evidently in favor of our probing to the formation mechanism of
urinary calculus in human body.
2.1 CaC2O4 crystallization controlled by chondroitin sulfate
Figure 1 showed the AFM images of crystallization process and aggregation of calcium oxalate accompanying the self-assembly of chondroitin sulfate film on mica. Calcium oxalate small balls scattered at first (Fig. 1 A) and gradually aggregated to form the crystal (Fig. 1 B and C), which was evidently induced or controlled by chondroitin sulfate due to that these crystals showed regular and some regular hexagon crystals were formed (Fig. 1 D, E and F) but at the control without chondroitin sulfate, the growth of calcium oxalate crystal or the crystal shape lacked the regularity (Fig. 2). These indicated that chondroitin sulfate had a great effect on the formation of calcium oxalate crystal.
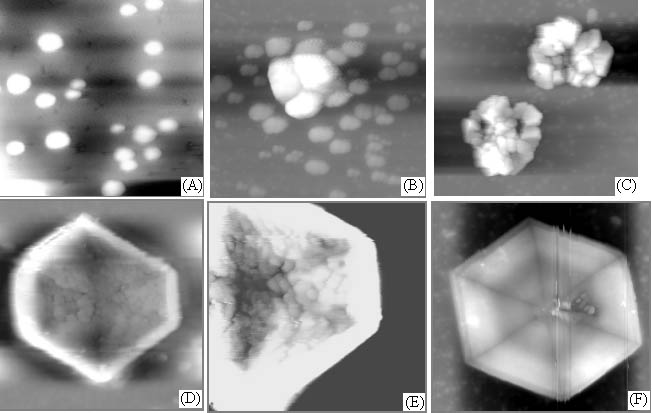
Fig. 1 AFM images of crystallization and aggregation of calcium oxalate accompanying the self-assembly of chondroitin sulfate film on mica. Scanning scopes: A. 5mm; B. 5mm; C. 15mm; D. 25mm; E. 15mm; F. 25mm.
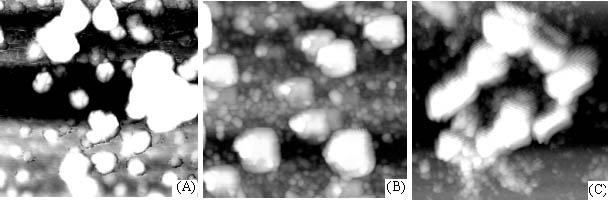
Fig. 2 AFM images of crystallization and aggregation of calcium oxalate in purified water on mica. Scanning scopes: A. 5mm; B. 1.7mm; C. 7mm.
2.2 Aggregation of CaC2O4
crystals on chondroitin sulfate film
After two days, crystals produced by the supersaturated solution of calcium oxalate on
chondroitin sulfate film were imaged with AFM and shown in the Figure 3. The crystals
aggregated to form many loops that relatively dispersed even on the film. These loops were
almost in the same shape, composed of hexagon, quadrilateral or irregular crystals, but
their diameters might display difference. The action of these calcium oxalate crystals was
evidently a Liesegang phenomenon [4], it indicated also that the aggregation of
calcium oxalate crystals was induced or controlled by chondroitin sulfate.
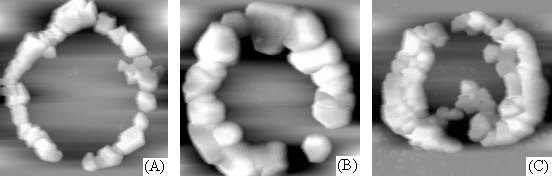
Fig. 3 AFM images of aggregation of calcium
oxalate crystals on chondroitin sulfate film.
Scanning scopes: A. 16mm; B. 10mm; C. 14mm.
The three-dimensional
image to the loop and its height curve map were shown in Figure 4. It showed that the
crystals size and their arrangement in the loop were relatively regular, which explained
also the affecting role of chondroitin sulfate to the crystallization and aggregation of
calcium oxalate.
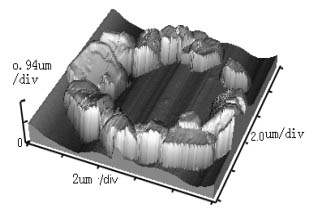
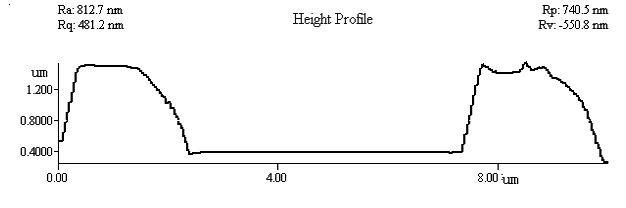
Fig. 4 Three-dimensional AFM image of Fig. 3
B and its height curve map.
After further scanning in
small scope at the center of the loops (Fig. 5), some calcium oxalate small balls were
found to be embedded in the meshes formed by chondroitin sulfate film itself and not to
participate the loop construction.
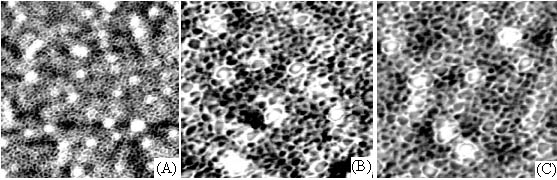
Fig. 5 AFM images of calcium oxalate crystal embedded in the meshes of chondroitin
sulfate film. Scanning scopes: A. 2.5mm; B. 1.5mm; C. 1.2mm.
3. DISCUSSION
From this study, the crystallization of calcium oxalate and its aggregation are
evidently related to the self-assembly of chondroitin sulfate molecules or its film.
Although this study cannot explain how the chondroitin sulfate molecules induce the
crystallization of calcium oxalate and its aggregation, or the chemical reaction between
them, based on the property of chondroitin sulfate molecule that is an acid
mucopolysaccharide with electronegative due to the existence of a lot of anion groups,
such as hydroxy, carboxy in the molecule, it may adsorb calcium cation at the specific
place [5] and favor the formation of the crystal core [6]. In
addition, the interaction between calcium cation and oxygen or nitrogen atom in
chondroitin sulfate molecule can fix calcium oxalate at the specific point on the film.
These form the foundation to induce or control the formation and growth of calcium oxalate
crystal. These controlled growth include the formation in size, shape and structure of the
crystal. The comparison in the growth of the crystal between the sample (Fig. 1, calcium
oxalate add chondroitin sulfate) and the control (Fig. 2, calcium oxalate add water)
supports this explanation.
As the formation of mature crystal with certain size, it begins moving
on the film driven by the diffusion force. During the moving, the crystal is also
controlled or affected by the film owing to the interaction between crystal and
chondroitin sulfate molecule. It is the chemical interaction and diffusion force, we
consider, that drive the crystals to contact and connect each other, and aggregate
regularly to form Liesegang loop. However, from our studies, the formation of Liesegang
loop is related to the concentrations of calcium oxalate and chondroitin sulfate. Suitable
concentration of chondroitin sulfate and supersaturated calcium oxalate are two key
conditions to form Liesegang loop. When the concentration of chondroitin sulfate is just
fit for forming reticulation (1.0mg/mL), it favors the formation of Liesegang loop of
calcium oxalate. But if the concentration of calcium ion is not enough, Liesegang loop
cannot form even if the concentration of chondroitin sulfate is suitable. The
concentration relationship between calcium oxalate and chondroitin sulfate for Liesegang
loop formation explain the complex process for the aggregation of mineral crystal induced
or controlled by organic matrix, or the relatively strict condition to form urinary
calculus in life body. These may mean that not only need enough mineral constituent but
also the organic matrix has to be suitable for the formation and growth of calculus in
life body.
On the other hand, those too small balls of calcium oxalate cannot move
to aggregate due to they have been embedded in the meshes of the film as shown in Figure
5. These mean the existence of interaction between crystals and chondroitin sulfate
molecules, and also that the crystal size is too small, the diffusion force of them is too
weak to move on the film. This is to say that it needs enough concentration for mineral
constituent for their aggregation in organic matrix system.
This study has presented a formation process of calcium oxalate mineral
crystal and its characteristics with the existence of chondroitin sulfate organic matrix
that is an important component of human urine, and also has given out the concentration
relationship between calcium oxalate and chondroitin sulfate for the formation of
Liesegang loop. These may help us to understand the formation process of urinary calculus
in human body. Evidently, more detailed studies are needed due to the complex process for
the formation and growth of mineral crystal induced or controlled by organic matrix
although our study has started the step on this direction.
4. CONCLUSIONS
Chondroitin sulfate can induce or control the formation and growth of calcium oxalate
crystal. These crystals may aggregate to form Liesegang loop on chondroitin sulfate film,
but the formation of Liesegang loop needs suitable concentration of chondroitin sulfate
and supersaturated calcium oxalate. The affecting role to calcium oxalate crystal by
chondroitin sulfate is ascribed to their interaction produced mainly by calcium ion with
those functional groups in chondroitin sulfate molecule.
5. EXPERIMENTAL
Chondroitin sulfate was purchased from Sigma, and 0.25g of the compound was weighed
and dissolved in 250 ml purified water. Calcium oxalate (in AR) of 0.6mol was made. A
sample of 5
For the investigation of the action of these crystals on the film, due to that the crystals on the film in the treatment above was too dilute, so that the 5mL of supersaturated solution of calcium oxalate was put on the chondroitin sulfate film, imaged with AFM after two days.
AFM (Autoprobe CP Research, THERMO MICROSCOPE, USA) was performed with a APSC-0100 scanner, Si3N4 probe needle, microarm length 180mm, force constant 3.2 N/m, imaged with tapping mode. All images were treated with the Flatten Auto to remove the low-frequency noise in the scanning direction. REFERENCES
[1] Heywood B R, Mann S J. Am. Chem. Soc., 1992, 114 (12): 4681-4686.
[2] Heywood B R, Mann S. Langmuir, 1992, 8: 1492-1498.
[3] Walsh D, Mann S. Nature, 1995, 377: 320-323.
[4] Soloway R D, Wu J G, Xu D F. Berlin Heide-berg, 1990, p35.
[5] Grenshaw M A. Biomineralization, 1972, 6, 6-11.
[6] Lochhead M J, Letellier S R, Vogel V. J. Phys. Chem., 1997, 101B, 10821-10827.
¡¡