http://www.chemistrymag.org/cji/2006/085036pe.htm |
May 10, 2006 Vol.8 No.5 P.36 Copyright |
Electrochemical study of the inhibition of bis-diisobutylaminomethyl-urea for the atmospheric corrosion of mild steel
Zhang Daquan, Gao Lixin, Zhong Lina, Zhou Guoding(Electrochemical Research Group, Shanghai University of Electric Power, Shanghai 200090, China)
Abstract The
inhibition of bis-diisobutylaminomethyl-urea (BDMU) for the atmospheric corrosion of mild
steel was investigated by volatile inhibiting sieve test (VIS) and electrochemical
measurements. Electrochemical impedance spectroscopy of a vapor phase corrosion inhibitor
monitor cell (VpCIM) was applied to study the effect of BDMU on the corrosion inhibition
of mild steel under thin electrolyte layer. The results show that BDMU has good protection
effect for steel. It suppressed the anodic reaction of steel electrode and rendered the
Ecorr to more positive direction. Reflected Fourier transform infrared (FT-IR)
spectroscopy was used to characterize the adsorption of BDMU on steel surface.
Keywords Steel; Bis-diisobutylaminomethyl-urea; Vapor phase
corrosion inhibitor; Electrochemical impedance spectroscopy (EIS);
1. INTRODUCTION
The use of vapor-phase corrosion inhibitors (VpCIs) is one of the most effective and
convenient methods for preventing metallic articles and equipment from corrosion during
transport and storage. There are numerous investigations on corrosion inhibitions by
aliphatic amines, alicyclic amines, and their salts as VpCIs for various industrial metals
and alloys. Among them, cyclohexyl ammonium carbonate (CHAC) and dicyclohexyl ammonium
nitrite (DICHAN) are the most commonly used VpCIs and have been used in industry for
several decades [1, 2]. However, the disadvantage of CHAC and DICHAN is their
toxicity. An attempt was made to develop new environment-friendly organic compounds as
VpCIs. Saurbier suggested toluylalanine as an effective temporary inhibitor of
steel in wet atmosphere [3]. Vuroinen reported a series
of morpholine Mannich base derivatives as vapor-phase corrosion inhibitors [4].
Some 6-methoxy-aminobenzothiazole derivatives were also used by Quraishi et al. to inhibit
the metal corrosion under vapor-phase conditions [5].
A VpCI needs to be a volatile compound that can be capable of forming a
relatively stable bond at the interface of the metal. Thus, the vapor pressure and its
volatile corrosion-inhibiting ability are the two very important properties for VpCIs [6].
Urea is one of the main ingredients of corrosion inhibitor formulations for many volatile
corrosion-inhibiting works. It is safe and cheap. But the disadvantage is its too high
vapor pressure to give lasting protection [7]. For this reason, work on urea
derivatives having greater inhibitory activity than that of the parent urea molecule is
desirable. In this paper, bis-diisobutylaminomethyl-urea (BDMU), which has a urea moiety
and two diisobutylamino moieties, was developed as a novel VpCI. The molecular structure
is described in Fig. 1. It has bulry molecular size and more N atoms in its molecule, and
therefore may have larger coverage on the metal surface and provide better inhibition
effect. Inhibition of mild steel corrosion by a BDMU film forming on the metal surface was
studied by a volatile inhibiting ability test and electrochemical measurement. Its
adsorption on the mild steel surface was investigated by reflected FT-infrared
spectroscopy.
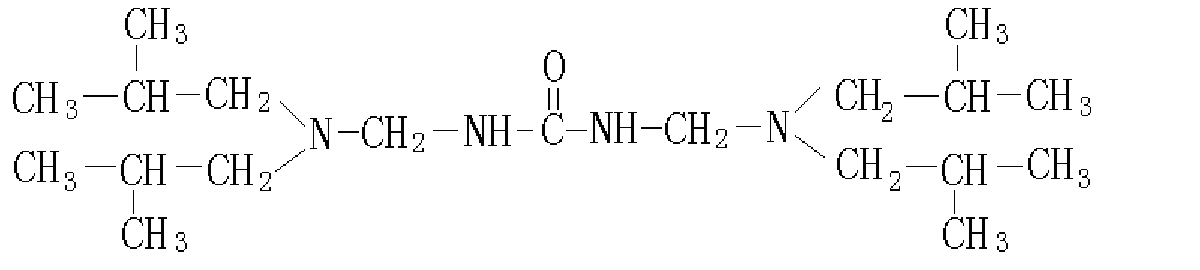
Fig.1 Molecular structure of
Bis-diisobutylaminomethyl-urea (BDMU)
2. EXPERIMENTAL
2.1 Materials and Apparatus
Mild steel strips were used for the corrosion test and electrochemical measurements. The
composition of the mild steel is given in%: C≤0.15;
Mn 0.20-0.45; P≤0.03; S≤0.035 and Al≥0.02. A vapor
phase corrosion inhibitor monitor cell (VpCIM) was used to obtain electrochemical data
with thin absorbed moisture layers according to a method developed by Bastidas et al. [8].
Electrochemical impedance spectroscopy was performed by using a PARC M283 potentiostat
(EG&G), PARC Model 1025 frequency response analyzer. The inhibitor, BDMU, was
synthesized according to the procedure reported in our earlier publication [9].
Briefly, a mixture of diisobutylamine and formaldehyde was stirred in ethanol solution for
1 hour. Urea (according to the reaction mole ratio) was dissolved in ethanol and was
slowly added to the above mixture. The reaction solution was stirred and refluxed for 2
hours. On cooling, the white precipitate of BDMU was collected and crystallized from
ethanol.
2.2 Procedures
2.2.1 Vapor phase corrosion inhibition test
The volatile inhibiting sieve test (VIS), was conducted to evaluate the inhibition effect
of BDMU. Each test was carried out with three specimens at the same time to give
reproducible results. Plates of steel were cut to shape (50mm×25mm×1.5mm) and a hole was
drilled in each for suspending the sample by a nylon thread. The samples were ground with
SiC paper to 1000 mesh and were then rinsed in alcohol before drying at room temperature.
The final geometrical area was 25cm2. The VIS test was conducted by suspending
the specimens in a 250 cm3 conical flask with a tight-fitting rubber cork
containing a small dish. The VpCI is dispersed in this dish. The specimens with freshly
prepared surface were mounted on the flask with and without 1.0g inhibitor, respectively.
The conical flasks were placed in an electrical hearted oven at 50±2℃ temperature. After 2 hours inhibitor film-forming period, 15cm3
deionized water was added. The electrical hearted oven was heated for 8 hours every day,
and then it was shut down for 16 hours. After corrosion test for 7 days, samples were
removed for visual inspection and mass loss determinations. The loose segments of the film
of the corroded sample were removed by hard rubber and rinsed in deionized water.
Corrosion rates and inhibitor effectiveness are calculated by means of the following
equations.
CR=(W
IE%=[(CR1-CR2)/CR1]×100% (2)
where CRs is in g m-2 h-1; A is the specimen area (in m2); W0 is original weight of the specimen, and W1 is specimen weight (in g) after the immersion period, T is the immersion period (in h), and CR1 and CR2 are the corrosion rates without and with an inhibitor, respectively.
2.2.2 Electrochemical measurements
Electrochemical measurements were conducted both in stimulated atmospheric corrosion water
and under thin electrolyte layer. A three-electrode cell, consisting of a mild steel rod
working electrode (WE), a platinum foil counter electrode (CE), and a saturated calomel
electrode (SCE) reference electrode was used for solution electrochemical measurements.
The WE was mechanically polished on wet silicon carbide (SiC) paper (grades 120, 600, and
1,200), rinsed with redistilled water, degreased with acetone and ethanol, and dried at
room temperature. The WE was introduced into an epoxy resin holder exposing a 1cm2
surface to the solution. The solution was prepared using redistilled water and containing
Cl- 0.1kg/m3, HCO3-0.1kg/m3, SO42-
0.1kg/m3, respectively. These anions were used as their sodium salts. As for
Potentiodynamic polarization curves test, the potential changed up to +250mV around
open circuit potential at speed of 1mV/s. The EIS experiments were performed at open
circuit potential over a frequency range of 0.05 Hz to 100 kHz. The sinusoidal potential
perturbation was 5 mV in amplitude. The potential values reported here were versus SCE.
All measurements were conducted in a non-stirred electrolyte. The cell was opened to the
laboratory air and the measurement was conducted at room temperature.
A vapor phase corrosion inhibitor monitor cell (VpCIM) was used to
obtain electrochemical impedance data with thin electrolyte. VpCIM consisted of 20 foils
of mild steel (80.0mm×8.0mm×0.5mm) and each steel plates was separated by a 170mm thick layer of an insulating sheet.
Alternate steel foils were connected to establish a 2-electrode configuration technique [10].
A block of 10 steel foils acted as the working electrode, and the other block of 10 steel
foils served as the reference electrode and the counter electrode. The above ensemble
(steel foils and insulating sheets) was encapsulated in an epoxy resin. One side of the
ensemble was polished with emery paper of different grades (#1, #4 and #6), degreased with
alcohol, and dried. The VpCIM was placed upon a 300 mL-beaker, which contained a lid of 5g
inhibitors. The edges of the polished steel foils faced down to the lid. After a specific
period of film-forming time of the inhibitor, the VpCIM was then covered with a filter
paper saturated by stimulated atmospheric water. The VpCIM was placed in a vessel at a
relative humidity of 90%. The EIS experiments were done according to a process described
as above.
2.3 FT-Infrared Reflection Test
The steel specimens of 5mm×5mm×1mm were abraded with silicon carbide (SiC) paper and
polished with diamond paste to 1mm. The samples were washed with acetone and isopropanol of
analytical grade and then immediately transferred to a desiccator. The samples were moved
to a vessel for BDMU treatment. After 8 hours inhibitor film-forming period, the
BDMU-treated steel samples were transported for FT-Infrared Reflection Absorption Spectra
analysis. A background spectrum was recorded for a sample without BDMU treatment. A Nexus
470 FT-IR spectrometer (Niclet) was connected for FT-IR surface analysis. The infrared
light from FTIR spectrometer bench was directed on the sample via plane and spherical
windows and strikes the sample surface 800 from the surface normal. The reflect
spectra were obtained by using p-polarized light at a resolution of 4 cm-1. All
subsequent spectrums were recorded in absorbance units (Rt/R0×100%),
where Rt is the reflected intensity of the BDMU-treated sample surface and R0
is the reflectivity of the background spectrum. The FT-Infrared adsorption spectrum of
BDMU with a potassium bromide (KBr) disc was also recorded for comparison.
3 RESULTS AND DISCUSSION
3.1 Volatile corrosion inhibition test
Visual inspection is the criterion for the vapor phase corrosion inhibition test. After
VIS test, the corrosion rate and inhibition effectiveness for the BDMU film-forming
specimens were 23.8 mg·m-2·h-1 and 84.6%,
respectively. This shows that BDMU can volatilize and adsorb on the mild steel surface to
protect the steel.
3.2 Electrochemical measurements in stimulated atmospheric corrosion water
Potentiodynamic polarization curves are shown in Fig. 2 for freshly polished mild steel
electrode after 2 hours immersion in stimulated atmospheric corrosion water.
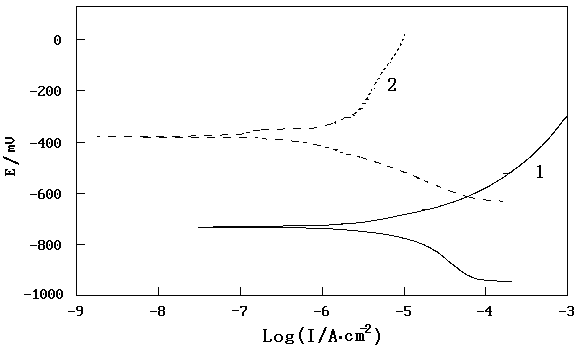
Fig. 2 Potentiodynamic polarization curves for steel
electrode after 2 hours immersion in simulated atmospheric corrosion water(1-blank,2- 0.2 % BDMU)
The anodic curve for the
steel electrode in stimulated atmospheric corrosion water exhibits an active dissolution
property. The mechanisms of anodic process of steel in electrolyte solutions are [11]:
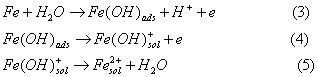
The cathodic portion of the polarization
curve is a composite and represents oxygen reduction. The cathodic corrosion reaction of
steel is:
![]()
The total corrosion reaction of steel in
aqueous solutions is as follows [12]:
![]()
![]()
It is clear that BDMU renders Ecorr
more possitive to –417.0mV for the steel electrode
in stimulated atmospheric corrosion water. In the presence of BDMU, both the cathodic and
anodic current densities were greatly decreased near Ecorr region. BDMU
decreases the anodic reaction rate more strongly than the cathodic reaction rate. This
indicates the effective corrosion inhibition by BDMU in stimulated atmospheric corrosion
water.
Fig. 3 shows Nyquist plots of a steel electrode in stimulated
atmospheric corrosion water with and without BDMU.
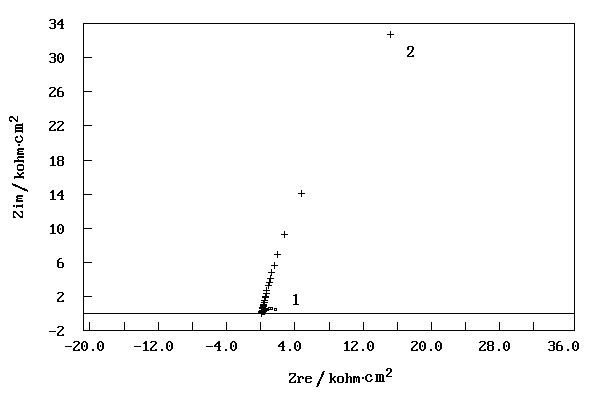
Fig. 3 Nyquist plots for steel electrode after 2 hours
immersion in simulated atmospheric corrosion water(1-blank,2- 0.2 % BDMU)
The corrosion resistance of each of the
samples was determined by Rp. Rp is given by [13]:
![]()
where Re represents the real part of the complex faradaic
impedance, and ω corresponds to the angular velocity
of the AC signal (w=2pf,where
f is frequency, Hz). Rp values were obtained by fitting the experimental
Nyqusit data to a simple semicircle and extrapolating to Zim=0. Comparing the
two Nyquist diagrams in Fig.3, it is observed that the size of the semicircle without BDMU
is far smaller than that with 0.2 % BDMU. This suggests that BDMU has excellent protection
effect for corrosion of mild steel in simulated atmospheric corrosion water.
3.3 Electrochemical impedance measurements under thin electrolyte layer
The atmospheric corrosion of mild steel is an electrochemical process that conducted under
thin electrolyte layer. The thin electrolyte layer was formed by adsorption, condensation
or precipitation of humidity in the air. A VpCIM was used to investigate the protection of
BDMU for mild steel under thin electrolyte layer. Fig. 4 shows Nyquist plots for a VpCIM
obtained with and without a period of BDMU film-forming.
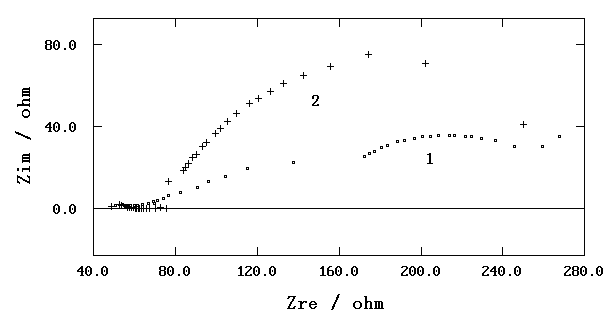
Fig. 4 Nyquist plots for a VpCIM under simulated
atmospheric corrosion water layer (1-blank, 2-after 4 hours BDMU film-forming)
The electrode impedance
with BDMU film-forming was higher than that without BDMU film-forming. The inhibition
effect of BDMU for mild steel was also proved under thin electrolyte layer.
3.5 FT-Infrared Reflection Test
As described above, infrared reflection absorption spectrum of steel specimen was obtained
after 8 hours BDMU film-forming periods. The infrared absorption spectrum of BDMU with a
potassium bromide (KBr) disc was also measured for comparison. Table 1 enumerated the
fundamental frequencies of the bands of BDMU and those of the corresponding reflection
spectra recorded after 8 hours exposure up to BDMU for film-forming periods.
Table 1 Frequencies(cm-1) of FT-IR spectra recorded on steel after BDMU film-forming periods
Bands |
Inhibitor (BDMU) |
Surface of steel |
| n(C-N) | 1364 |
1294 |
n(C=O) |
1663 |
1605 |
The adsorption of BDMU on
steel surface is detected by the reflectance spectrum of steel surface. The bands around
1605cm-1 and 1294cm-1 were shifted about 58cm-1 to 70cm-1
compared to the infrared absorption spectrum for BDMU. This effect can be explained by
assuming the interaction of Fe with N, O heteroatoms [14]. There are four N
atoms and one oxygen atom in BDMU molecule. BDMU is capable of entering into interaction
with the metal, for it has one pair electron on the nitrogen atom and two pair electrons
on the oxygen atom. The metal surface usually becomes an electron acceptor. The formation
of a donor-acceptor bond between BDMU and metal hinders the transfer of a metal ion from
the metal lattice into solution. The mode of BDMU bonding on the steel surface is
suggested in Fig. 5. BDMU can attach itself to steel surface through weak chemical bonding
and forms an adsorbed monolayer to shield this interface from penetration by corrosive
agents such as water, erosive ions ( Cl- or SO42- ),etc. The adsorbed monolayer may change the rate of electrochemical
reactions like the dissolution of metal or the reduction of oxygen.
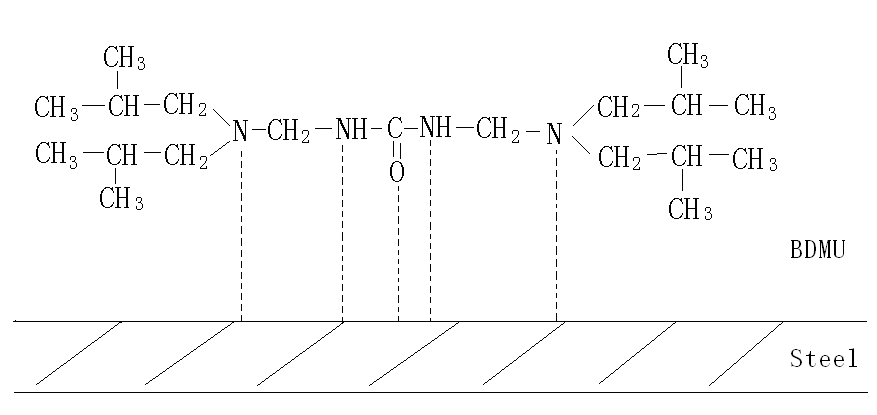
Fig. 5 Schematic of BDMU absorbed on the steel surface
4. CONCLUSIONS
Bis-diisobutylaminomethyl-urea (BDMU) has been proved to be a good vapor phase corrosion
inhibitor for the mild steel. BDMU suppressed the anodic reaction of steel electrode and
renders the corrosion potential to more positive direction. Infrared reflection absorption
spectrum suggests that interaction exists between Fe and N, O heteroatoms of BDMU
molecule.
Acknowledgments Supports from the National Natural Science Foundation of China (20576069) and from Shanghai leading academic discipline project (P1304) are gratefully acknowledged.
REFERENCES
[1] A. Subramanian, M. Natesan, V. S. Muralidharan, et al. Corrosion, 2000, 56 (2): 144.
[2] E. Stupnisek-Lisac, V. Cinotti, D. Reichenabac. J. Appl. Electrochem., 1999, 29: 117.
[3] K. Saurbier, V. Mendorf, J.W. Schultze, et al. Corros. Sci. 1992, 33 (9): 328.
[4] E. Vuorinen, P. Ngobent, G. H. Vander, et al. Br. Corros. J., 1994, 29 (2): 120.
[5] M.A. Quraishi, D. Jamal. Corrosion, 2002, 58 (3 ): 387.
[6] L.R.M. Estevao, R.S.V. Nascimento. Corros. Sci., 2001,43 (11 ): 1133.
[7] E. Vuorinen, E. Kalman, W. Focke. Surf. Eng. 2004, 20 (4): 281.
[8] J. M. Bastidas, E.M. Mora, S. Feliu. Werkst. Korros. ,1990, 41(1 ): 3.
[9] Z. X. An. Study on inhibition performance and
mechanism of organic polyamine volatile corrosion inhibitors (Master Degree thesis, Shanghai University, Shanghai, China, 2004.
[10] L.X. Gao, D.Q. Zhang. Mater. Prot., 2000, 33 (6): 39.
[11] P. Li, J. Y. Lin, K. L. Tan, et al. Electrochimica Acta, 1999, 42 (4): 605.
[12] A. Leng, M. Stramann, Corro. Sci. 1993, 34 (10): 1657.
[13] R. Gasparac, E. Stupnise-Lisac.Corrosion., 1999, 55 (11) :1031.
[14] D. Q. Zhang, L. X. Gao, G. D. Zhou. Corrosion, 2005, 61 (4): 392.
张大全,高立新,钟丽娜,周国定
(上海电力学院电化学研究室,上海200090,中国)
摘要 采用气相防锈筛选试验和电化学测量方法研究了双二异丁基胺甲基脲(BDMU)对碳钢大气腐蚀的抑制作用。通过气相缓蚀剂监测电池的电化学阻抗谱,考察了BDMU对薄层电解液下的碳钢腐蚀的作用特性。结果表明BDMU对碳钢有很好的保护作用,它的加入强烈抑制了腐蚀电化学阳极过程并且使腐蚀电位正移。结合红外反射光谱研究了BDMU在碳钢表面的吸附作用。
关键词 碳钢;双二异丁基胺甲基脲;气相缓蚀剂;电化学阻抗