http://www.chemistrymag.org/cji/2006/085037ne.htm |
May 16, 2006 Vol.8 No.5 P.37 Copyright |
(Institute of New Drug Research, Jinan University College of Pharmacy, Guangzhou 516032, China )
Received Mar. 13, 2006.
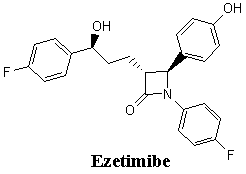
Abstract Ezetimibe, a novel and potent
cholesterol lowering agents, is called the cholesterol absorption inhibitors (CAIs).
Intermediate of Ezetimibe was synthesized by the reaction of Staudinger under microwave
irradiation. This method was simple and fast. The structure of the product was confirmed
by IR, 1HNMR and MS spectra.
Keyword microwave chemistry; synthesis; intermediate
1 INTRODUCTION
High cholesterol is the best known of all the many threats to a healthy
heart. Cardiovascular diseases are the first cause of death in majority of the developed
countries. Although there have been significant progresses in the treatment of
cardiovascular diseases during the last several years, there are still no efficacious cure
for most forms of human cardiovascular diseases, including heart attack, stroke and so on.
Ezetimibe (SCH 58235, 1-(4-fluorophenyl)-3(R)-[3-(4-fluorophenyl) -
3(S) - hydroxypropyl] - 4(S) - (4 - hydroxyphenyl)-2-azetidinone) is a novel and potent
cholesterol lowering agent called the cholesterol absorption inhibitors (CAIs) that
selectively inhibits cholesterol absorption across the intestinal wall. Ezetimibe potently
inhibits the absorption of biliary and dietary cholesterol from the small intestine
without affecting the absorption of fat-soluble vitamins, triglycerides or bile acids. It
was reported from clinical studies that Ezetimibe significantly decreased plasma LDL
cholesterol levels and increased plasma HDL levels with an excellent tolerability profile.
Ezetimibe is unique in the way it helps block the absorption of cholesterol that comes
from food, a different way to fight against cholesterol. [1-4]
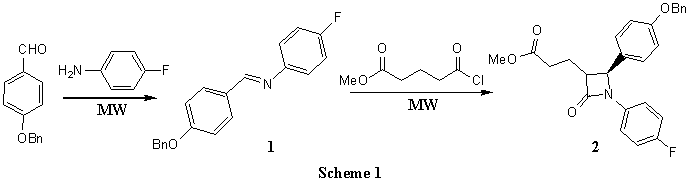
Many ways have been developed to
synthesize Ezetimibe, there have two methods mainly in the reported. luteratures[5-8] However, synthesis of intermediate comprising β-lactam(2) is the most
important in each approach to synthesize Ezetimibe. In this paper, we wish to report
microwave-assisted rapid synthesis of intermediate of Ezetimibe. The commercially
available 4-fluorobenzenamine and 4-(benzyloxy)benzaldehyde were mixed in a conical flask
catalyzed by microwave to afford N-(4-(benzyloxy)benzylidene)-4-fluorobenzenamine(1)
in 90%. β-lactam was
prepared via the ketene-imine condensation. Compound 1 and methyl glutaryl chloride
were catalyzed by microwave through Staudinger reaction, affording compound 2 (Scheme
1).
2 EXPERIMENTAL
Melting points were uncorrected and were measured with micro-melting point apparatus
XT-4. IR spectra (KBr) were obtained on a Thermo Nicolet Nexus 470 FT-IR spectrometer.
1H NMR spectra were determined on a Varian Mecurry 300 spectrometer using CDCl3
as solvent and tetramethylsilane (TMS) as internal reference. Mass spectra were
recorded using a Thermo Finigann Trace 2000 GC-MS mass spectrometer. Microwave irradiation
was carried out with commercial LG domestic microwave oven (1000W).
2.1 Preparation of N-(4-(benzyloxy)benzylidene)-4-fluorobenzenamine(1)
A mixture of 4-fluorobenzenamine (0.2ml,2.1mmol)
and 4-(benzyloxy)benzaldehyde (0.45g, 2.1mmol) in a conical flask was introduced into the
microwave oven and irradiated for 2min (output power at 50%). After cooling, the solid was
recrystallized from ethyl acetate/petroleum ether to provide (0.58g, 90%) of the title
compound as a yellow lamellar crystal. Mp: 136-138℃.
IR (KBr cm-1) :1627, 1603, 1504, 1216, 834, 739. 1HNMR (CDCl3,
ppm)d: 8.3(s,1H,N=CH),7.8(d,2H,Ar), 7.4-6.6(m,11H,Ar),
5.1(s,2H,CH2). MS(EI):m/z 305(M+).
2.2 Preparation of
trans-methyl-3-(3-[2-oxo-4-(4-benzyloxyphenyl)-1-(4-fluorophenyl)-azetidinyl]propanoate
(2)
A mixture of compound 2 (0.5g, 1.6mmol), methyl glutaryl chloride (0.3ml,2.1mmol) and n-tributylamine (1ml,4.2mmol) in a conical flask was
introduced into the microwave oven and irradiated for 2min (output power at 50%). After
cooling, the mixture was purified by flash silica gel chromatography (ethyl
acetate/petroleum ether/triethylamine,9/1/0.5), affording (0.21g,30%) the title compound as a white needle crystal. Mp:84-86℃. IR(KBr cm-1): 2949, 1732, 1611, 1510, 1438,
1387, 1239, 1176, 1010, 837, 746. 1HNMR(CDCl3,ppm) d:
7.4-7.1(m,11H,Ar),
6.9(q,2H,Ar),5.03(s,2H,CH2Ph),4.6(d,1H,J=2.1Hz,NCHAr),3.6(s,3H,OCH3),3.1(m,1H,C(O)CH),
2.6-2.4(m,2H, C(O)CH2),2.2(q,2H,CH2CH). MS(EI):m/z 433(M+).
3 RESULTS AND DISCUSSION
Microwave irradiation is becoming an
increasingly popular method of heating which replaces the classical one as it proves to be
a clean, cheap, and convenient method. Often, it affords higher yields and results in
shorter reaction time. This method of heating has been extended to almost all areas of
organic chemistry.[9] Under Staudinger reaction in the normal condition, the
product’s
configuration and yield appear to be affected by several factors, including solvent,
temperature, base, and the sequence of dripping of reagents. Although the acyl
chloride-imine reaction is a well established method for the synthesis of b-lactam,
modifications which improve the yield, shorten synthetic time and decrease the cost are
important in light of the biological significance of β-lactam. In this paper, intermediate of Ezetimibe was
synthesized under microwave irradiation. It was demonstrated again that microwave reaction
have many virtues such as convenience, cleanness and speediness. This approach to
synthesize Ezetimibe would be suitable for manufacture in industry.
However, microwave
reaction was appeared to be sensitive to a significant factor, the power of microwave
irradiation. In the microwave synthetical experiment, we selected low, middle and high
power of microwave irradiation respectively (the commercial LG domestic microwave oven
only have three choices) and irradiated for 2 min. Fig.1 is the 1HNMR compared
picture of the crude product b-lactam characteristic absorption band in different power of
microwave irradiation. Form the picture it could be seen that the yield of trans-product
increased along with the power of microwave irradiation enhanced. Probably with the power
of microwave irradiation enhanced, the heat effect would be reinforced which accords with
elevated temperature would be propitious to the yield of trans-product in normal reaction.
[10] However, the product was inclinable to coking and difficult to
purification and by product increasing when the power of microwave irradiation enhanced.
Due to the complexity of Staudinger reaction, the condition of synthesis under microwave
irradiation would be optimized in the future.
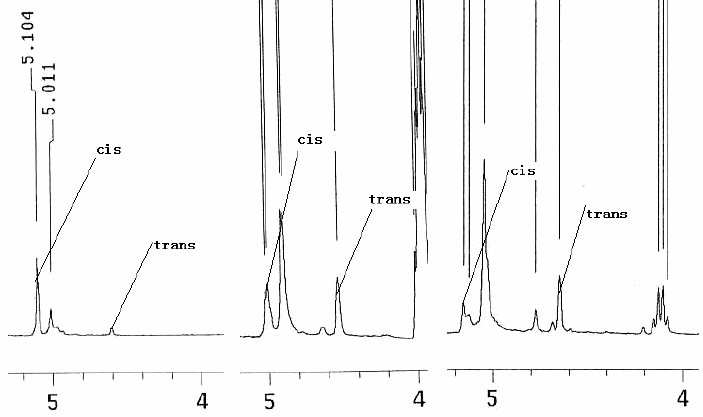
Fig.1 1HNMR of
the crude product β-lactam
characteristic absorption band in different power of microwave irradiation (from left to
light is low, middle, high)
4 CONCLUSIONS
In the paper,
trans-methyl-3-(3-[2-oxo-4-(4-benzyloxyphenyl)-1-(4-fluorophenyl)-azetidinyl] propanoate
was successfully synthesized under microwave irradiation. From what has discussed above, a
rapid and efficient method for the preparation of intermediate of Ezetimibe has been
provided, which has the characteristics such as its simplicity in operational, high
yields, short reaction time and low cost. This method will be better than the existing
ways.
Acknowledgement Thank for the help of Simon Wang in Wuhan University.
REFERENCES
[1] Fu X, McAllister T L, Thiruvengadam T K, et al. Tetrahedron Lett.,
2003, 44 (4): 801.
[2] Bays H E, Moore P B, Drehobl M A, et al. Clin. Ther., 2001, 23 (8): 1209.
[3] Davis H R. International Congress Series, 2004, 1262: 243.
[4] Clader J W. J. Med. Chem., 2004, 47 (1): 1.
[5] Wu G Z, Wong Y S, Chen X, et al. J. Org. Chem., 1999, 64 (10): 3714.
[6] Vaccaro W D, Sher R, Davis H R. Bioorg. Med. Chem., 1998, 6 (9): 1429.
[7] Rosenblum SB, Huynh T, Afonso A, et al. J. Med. Chem., 1998, 41 (6): 973.
[8] Duane A B, Mary A C, Martin S D, et al. Bioor. Med. Chem. Lett., 2002, 12 (3): 311.
[9] Corsaro A, Chiacchio U, Pistara V, et al. Curr. Org. Chem., 2004, 8 (6): 511.
[10] Barreau M, Commerçon A, Mignani S, et al. Tetrahedron, 1998, 54 (38): 11501.
杨兆琪
(暨南大学药学院新药研究所, 广州 510632)
摘要 Ezetimibe是一种新颖和有效的降胆固醇药物,称为胆固醇吸收抑制剂(CAIs)。在微波辐射的条件下,通过Staudinger反应合成了新型降血脂药Ezetimibe的中间体。此法比常规反应快而且操作简单,目标产物结构通过IR、1HNMR和MS表征。
关键词 微波化学;合成;中间体