http://www.chemistrymag.org/cji/2007/092008pe.htm |
Feb. 2,
2007 Vol.9 No.2 P.8 Copyright |
Si Chao1,2, Guo Hong 2,
Zhao Yuanqing 2, Huang Qilin 2, Hu Qiufen 2
(1School of Chemical Engineering, Sichuan University, Chengdu 610065; 2Department
of Chemistry, Yuxi Teacher's College, Yuxi, Yunnan, 653100, China )
Received Dec. 17, 2006.
Abstract A new method for the determination of trace amounts of mercury based on the reaction of Hg(II) with 6-mercaptopurine and the solid phase extraction of the complex on C18 membrane disks was developed. The 6-MP selectively reacts with Hg(II) to form a complex in the pH range of 5-8. This complex was preconcentrated by solid phase extraction with C18 disks. An enrichment factor of 100 was achieved. The molar absorptivity of the complex is 4.17 × 104 L.mol-1.cm-1 measured at 315 nm. The Beer's law is obeyed in the concentration range of 0.2-5.4 mg.mL-1. The relative standard deviation for eleven-replicated measurement of 0.024
mg.mL-1 is 1.5 %. The results are satisfactory.Keywords mercury; 6-mercaptopurine; preconcentration; solid phase extraction.
1. INTRODUCTION
Mercury is a serious environmental pollutant with toxic effects in all living organisms.
Therefore, the development of analytical methods for the determination of mercury is
important [1,2]. A serious problem encountered in the determination of mercury
is that target species are usually present in low concentration. The main species of
mercury in natural waters to be identified and determined are inorganic mercury (Hg2+)
and methyl mercury (CH3Hg+). The routine spectrophotometric methods
are often not sensitive or selective enough to determine low concentration of mercury ion
in environmental samples. Consequently, a preconcentration step is usually required. The
most widely used preconcentration methods are coprecipitation, ion exchange, solvent
extraction, flotation and solid phase extraction (SPE) [3-6]. Solid phase
extraction is an attractive technique that reduces solvent consumption and exposure,
disposal costs and extraction time for sample preparation [7]. 6-Mercaptopurine
(6-MP) is a biologically active molecule containing sulfur and nitrogen donor sites that
can form stable complex with mercury[8-9]. This study describes a procedure for
the determination of inorganic mercury in water samples using the solid phase extraction
technique. Several significant advantages of the present method includes, simplicity of
the operation, least interferences, excellent detection and avoiding the use of harmful
organic solvents.
2. EXPERIMENTAL
2.1. Apparatus
A UV-2401 Spectrophotometer (Shimadzu, Japanese) was used for all
absorbance measurements with a 1 cm quartz cell. Solid phase extractions were conducted on
C18 membrane disks, ENVI-18DSKTM [47 mm (diameter) × 0.6 mm (thickness) 30
2.2. Materials and Solutions
Analytical reagent grade chemicals were employed for the preparation of all solutions. Solutions were prepared using deionized water from a Nanopure water system (Millipore corporation, USA). Methanol Merck (Darmstadt, Germany) was used. A stock solution of mercury (1000 mg.mL-1, Hg(II) in 0.5 mol.L-1 HNO3) was prepared from mercury chloride (Darmstadt, Germany). Working Hg(II) standards were prepared daily by appropriate dilution of the stock solution. The selected reagent, 6-MP, was provided by Sigma-Aldrich (Steinheim, Germany). A solution of 1.0 ×10-3 mol.L-1 6-MP was prepared daily by dilution with the buffer solution. The 0.1 mol L-1 phosphate buffer solution was prepared by dissolving appropriate amount of sodium dihydrogen phosphate in 500 mL water, then adjusting the pH to 6 with sodium hydroxide solution and diluting to a volume of 1000 mL with water. Special care was taken in the preparation and handling of solutions and containers to minimize any possible risk of mercury contamination. Calibration flasks were left overnight in 10% (v/v) HNO3 and then rinsed thoroughly with ultra-pure mili-Q water before use to minimize exogenous metal contamination.
2.3. General Procedure
To a standard or sample solution containing no more than 13.5 mg of Hg (II) in a 250 mL of calibrated flask 25 mL of sodium dihydrogen phosphate-disodiumhydrogen phosphate buffer solution (containing 0.1 mol L-1 Na2EDTA) and 5 mL of 1.0 × 10-3 mol L-1 of 6-MP solution were added. The mixture was diluted to the volume of 250 mL and mixed well. After 10 min, the solution was passed through the C18 disk at a flow rate of 50 mL min-1. The mercury complex was retained on the disk. After the enrichment finished, it was desorbed from the disk with 2.5 mL of methanol (contain 0.5% KOH) at the flow rate of 5 mL min-1 in reverse direction. The absorbance of this solution was measured at 315 nm in a 1 cm cell against a reagent blank prepared in a similar way without mercury.
2.4. CVAAS Analysis
The CVAAS analysis was carried out with a Varian (Spectra AA-220) Atomic Absorption Spectrometer equipped with mercury hallow cathodic lamp and a vapor generator accessory (VGA 77) in a continuous system. The experimental conditions were as slit width, 0.5 mm; lamp current, 4 mA; wavelength, 253.7 nm; time constant, 5s; PMT voltage, 290 V.
3. RESULT AND DISCUSSION
3.1. Absorbance Spectra
The absorption spectra of 6-MP and its Hg(II) complex under the optimum conditions are
shown in Fig. 1. As seen, the spectra of the Hg (II)-6-MP complex have two maximums that
overlap with the maximum of the ligand. However, it does not interfere in determination of
mercury because the unreacted 6-MP would not retain on the C18 disk. The peak
at 315 nm is more practicable in real sample. Thus, the wavelength of 315 nm was used in
all subsequent absorbance measurements.
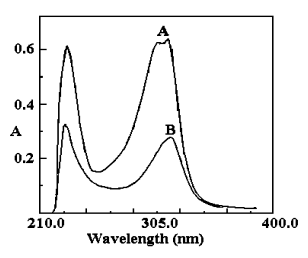
Fig. 1. Absorption spectra of 6-MP and its mercury
complex
(A) Hg (II)-6-MP complex against reagent blank (B) 6-MP in Methanol (contain 0.5%KOH)
3.2. Effect of the pH
The result indicated that the optimal pH for the reaction of Hg(II) with 6-MP was 5.0-8.0
as shown in Fig. 2. In acidic pHs formation of complex between Hg(II) and 6-MP is not fast
enough and in basic pH the complex would be solved easily in aqueous medium, In acidic pH
the selectivity is improved noticeably, therefore, the pH 6.0 was selected as the optimum.
A sodium dihydrogen phosphate- disodium hydrogen phosphate buffer solution of pH 6.0 was
recommended to control pH. The use of 10-50 mL of the buffer solution (pH 6.0) per 250 mL
of the final solution was found to give the maximum and stable absorbance. The use of 25
mL of the buffer solution is recommended.
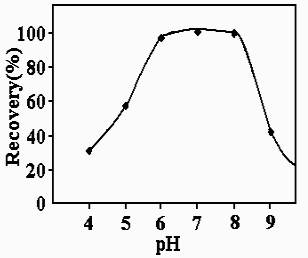
Fig. 2. Effect of pH on the percentage of recovery
3.3. Effect of 6-MP Concentration
The optimum amount of 6-MP for the quantitative extraction of Hg(II) was also investigated
as shown in Fig.3. From these results, the addition of about 5.0 mL of 1×10-3
mol L-1 of 6-MP solution has been found to be sufficient for a complete
reaction. Accordingly, 5.0 mL of 6-MP solution was added in all of further measurements.
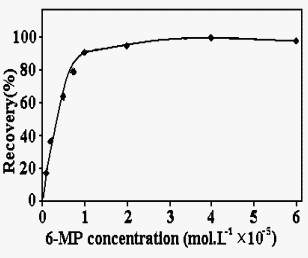
Fig. 3. Effect of 6-MP concentration on the percentage of recovery
3.4. Stability of complex
After mixing the reactants, the absorbance reaches its maximum within 6 min at room
temperature and remains stable for 12 h in aqueous solution. The complex is stable for at
least 24 h if extracted into the methanol (contain 0.5 % KOH).
3.5. Effect of Surfactant
The effect of surfactants was tested on the recovery of extraction by a cationic
surfactant (CTAB) an anionic surfactant (SDS) and a non-anionic surfactant (Triton-
X-100). None of them increase the absorbance markedly. Therefore, no surfactants were
added in the final procedure developed.
3.6. Solid Phase Extraction
Some experiments were carried out in order to investigate the retention of 6-MP and
its Hg(II) complex on the disks. It was found that the Hg(II)-6-MP complex was retained on
the disks quantitatively when it passes the disk as aqueous solution. The capacity of the
disk for the Hg(II)- 6-MP complex was determined as 18 mg in 250 mL of solution. In this
experiment, the disks have adequate capacity to enrich the Hg(II)-6-MP complex. In order
to choose a suitable eluent for the retained Hg(II)-6-MP complex, various organic solvents
were examined. It was found that pure organic solvents could not elute the Hg(II)-6-MP
complex from the disk quantitatively. Regardless of two types of sites of 6-MP (N,S), It
acts as a monodendate ligand through sulfur with mercury [10], therefore the NH
site is inactive in case of mercury. Moreover, in basic medium, the polarity of complex
increases owing to H releasing then the solubility of complex increase in polar organic
solvents like methanol. So, methanol (contain 0.5% of KOH) was selected as eluent.
Experiment showed that it was easier to elute the retained complex in reverse direction.
Methanol (2.5 mL contain 0.5% of KOH) was sufficient to elute the complex from the disk at
a flow rate of 5 mL min-1. Therefore, the volume 2.5 mL of eluent was chosen.
3.7. Calibration Curve and Sensitivity
The calibration curve showed that the Beer's law is obeyed in the concentration range of
0.2 - 4.8 mg Hg(II) per mL in the measured solution. The linear regression equation obtained
was: A = 0.1883 C (m
Table 1 Tolerance limits in the determination of 0.024 mg L-1 of Hg(II) with 6-MP (relative error ±5%)
| Ion added | Tolerated (g.L-1) |
| Na+, K+, CH3COO-, SO42-, PO43- | 40 |
| Ca2+, Ba2+, Mg2+, Bi3+ | 3 |
| Pb2+, Cu2+, Cd2+, Fe3+,Ni2+, Cr3+,Co2+ | 0.2 |
| Fe2+, Al3+,CO32-, Cl-, Ag-, Br- | 0.01 |
| I-, CN- | 0.001 |
3.8. Interferences
The selectivity of the proposed method was investigated by the determination 0.024 mg L-1
of Hg(II) in the presence of various ions within a relative error of ±5%. The tolerance
limits are listed in Table 1. According to these results, the method presents high
selectivity. The interference of Cu, Pb and Cd were eliminated successfully by the use of
EDTA, Furthermore, the formation of Ag(I)-6-MP complex also could not interfere in the
determination of mercury. Because the molar ratio of Ag complex is 1:1 while that of
mercury is 1:2 therefore, it would not retain on the C18 disk. In almost all
spectrophotometric methods for determination of Hg(II), Ag (I) is a serious interfering
cation, but this method presents a selective spectrophotometric method for determination
of Hg(II) without Ag(I) interference.
3.9. Application
In order to validate the methodology, the proposed method was applied to different water
samples for mercury determination. The waste water, river water and tap water were
collected from Kunming, Yunnan, P.R.China. The water samples were acidified by HNO3
and filtered through 0.45mm milipore membrane filters, prior to analysis. Along with the
samples, several known amount of Hg(II) were spiked to examine the reliability of the
method. The validity of the proposed method was confirmed by comparing the results
obtaining from the sample analysis with those obtained by CVAAS. The results of various
sample analysis are tabulated in Table 2.
Table 2 Determination of mercury in water
samples
Sample |
Hg(II) added |
Measured (m g.L-1) |
Standard recovery (%) |
|
Proposed method |
CVAAS |
|||
Waste Water |
0 |
5.20 ±0.11 |
0 |
|
16 |
21.3 ±0.17 |
16 |
97-102 |
|
32 |
37.4±0.15 |
32 |
98-101 |
|
River Water |
0 |
N.D. |
0 |
|
16 |
16.14±0.14 |
16 |
96-104 |
|
32 |
32.18±0.13 |
32 |
97-103 |
|
Tap Water |
0 |
N.D. |
0 |
|
16 |
16.07±0.07 |
16 |
94-102 |
|
32 |
32.09 ±0.11 |
32 |
98-105 |
|
4. CONCLUSIONS
The proposed solid phase extraction (SPE) method is a simple, rapid and high selective
method for separation, preconcentration and determination of mercury in different water
samples. Practically any of applied cations interferes with the proposed method; this
showed that the complexing agent is very selective toward Hg(II) in the presence of other
metal ions. This could be considered as an important advantage of both the ligand and the
proposed method. The working pH of this method is actually suitable for natural waters.
Furthermore, the enrichment factor of 100 was achieved with solid phase extraction by C18
disks. The detection limit of proposed method reaches 0.5 mg L-1, therefore the low
concentration of mercury could be determined in water samples with good results.
REFERENCES
[1] Yoshida M, Satoh M, Yasutake A, Shimada A, Sumi Y, Tohyama, C.
Toxicology, 1999, 139, 288-294.
[2] Li Y, Duang Z M, Sheng Q P, Wang J H. Chin.J.Environ.Health (Huangjing
Yu Jiankang Zhazhi). 2005, 22, 399-400.
[3] Kubova J, Neveral V, Stresco V. J. Anal. Atom. Spect, 1994, 9,
241-243.
[4] Hosseini M S, Hashemi-Moghaddam H. Anal. Sci, 2004, 20,
1449-1452.
[5] Tokman N, Akman S, Ozcan M. Talanta, 2003, 59, 201-205
[6] Zhang P, Wang X N, Wu Y J, Liang M H. Chin.J.Environ.Chem (Huangjing Huaxue), 2005,
24, 718-720
[7] Chen J, Wang Z Y. Chin.J.Chromatogr (Sepu), 2006, 24, 447-450
[8] San B, Costa J M, Pereiro R, Sanz-Medel A. Anal. Chim. Acta, 2000, 419, 33-40
[9] San B, Costa J M, Pereira R, Sanz-Medel A. Anal. Chim. Acta, 2002, 451, 203-210
[10] Chifotides H T, Dunbar K R, Katsaros N, Pneumaticakis G. J. Inorg. Biochem. 1994, 55, 203-216
司 超1,2,郭 红2,周元清2,黄齐林2,胡秋芬2
(1四川大学化工学院,成都 610065;2玉溪师范学院化学系,云南玉溪 653100)
摘要 根据6-巯基嘌呤(6-MP)与汞的显色反应及C18 固相萃取盘对显色络合物的固相萃取,建立了一种测定痕量汞的方法。在pH为5-8范围内,6-MP与汞反应生成稳定络合物,该络合物可被C18 固相萃取盘萃取富集,富集倍数达100倍,富集后的洗脱液用光度法测定,体系λmax=315 nm,e=4.17 ×104 L.mol-1.cm-1。汞含量在0.2-5.4 mg.mL-1 内符合比尔定律,方法用于痕量汞的测定,结果令人满意。
关键词 6-巯基嘌呤;汞;固相萃取;光度法