http://www.chemistrymag.org/cji/2007/0930010ne.htm |
Mar. 2,
2007 Vol.9 No.3 P.10 Copyright |
Wang Junpu, Huang Limin, Zhang Wei
(College of Chemistry and Engineering, Lanzhou University, Lanzhou 730000,
China)
Received Jan. 9, 2007.
Abstract A facile synthesis of quino[1,2-c][1,3]benzoxazin-6-ones and quino[1,2-c][1,3]naphthoxazin-6-ones has been developed via Diels-Alder reaction of N-phenyl-N-acyliminium cations, produced from 4-hydroxy-3-phenyl[1,3]benzoxazin-2-one and 4-hydroxy-3-phenyl[1,3]naphthoxazin-2-one in the presence of BF3·OEt2, with olefins in moderate to good yields at ambient temperature.Keywords quino[1,2-c][1,3]benzoxazin-6-ones, quino[1,2-c][1,3]naphthoxazin-6-ones, 4-hydroxy-3-phenyl[1,3]benzoxazin-2-one, Diels-Alder reaction
1. INTRODUCTION
The chemistry of tetrahydroquinoline
derivatives has long been an area of intense interest for organic chemists due to the
presence of these scaffolds within the framework of numerous biologically active natural
products and pharmaceutical agents. Therefore, many new methods for the synthesis of
tetrahydroquinoline derivatives have been developed.[1] Recently we have
reported a new [4 + 2] cycloaddition reaction of cyclic N-phenyl-N-acyliminium
cations with olefins based on which a variety of new polycyclic tetrahydroquinoline derivatives has been
synthesized.[2] In this strategy, the N-phenyl-N-acyliminium
cations acted as 2-azadiene [C=C-N(C=O)+=C-] analogues. As the continuation of
this work, we present here an efficient synthesis of quino[1,2-c][1,3]benzoxazin-6-one and
quino[1,2-c][1,3]naphthoxazin-6-ones via Diels-Alder reactions of new N-phenyl-N-acyliminium
cations with olefins such as styrene (2a), a-methylstyrene (2b), anethole (2c), indene (2d).
Intermediate 3-phenyl[1,3]benzoxazin-2-onium cations were generated from
4-hydroxy-3-phenyl[1,3]benzoxazin-2-ones (1a-c) and
4-hydroxy-3-phenyl[1,3]naphthoxazin-2-one (1d) which were reported to have many
potential biological activities[3] such as antimicrobial, antimycobacterial,
antifungal, antihypotensive and antiinflamatory activeities.To the best of our knowledge,
only one paper was found to deal with the synthesis of this kind heterocycles by the
condensation of quinoline N-oxide with enamine of cyclohexanone to give
2-(2-quinolyl)cyclohexanone and subsequently reduction to produce
2-(2-tetrahydroquinolyl)cyclohexanol which then condensated with formylaldehyde.[4]
Our paper describes for the first time the synthesis of these heterocycles via
Diels-Alder reactions of N-phenyl-N-acyliminium cation with olefins.
2. RESULTS AND DISCUSSION
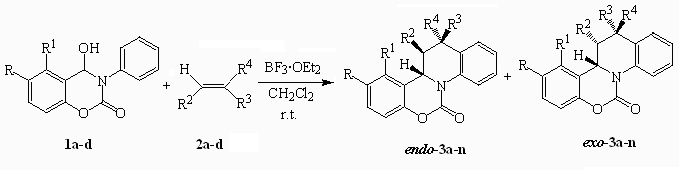
The [4+2] cycloaddition reactions of 1a-c
with olefins 2a-d were performed in the presence of BF3·OEt2
in anhydrous methylene dichloride at room temperature. The reaction were very quick
and efficient to afford the adduct 3a-n as a mixtures of endo and exo
isomers in moderate to high yields as shown in Scheme 1 and in Table 1. The endo
and exo isomers can be separated by column
chromatography or repeated thin layer chromatography and their configurations are assigned by 1H, 13C and 2D NMR
spectroscopy and confirmed by NOESY correlation.
Scheme 1
For example, the cis configuration of the C-12 and C-14 in endo-3a was assigned by the large vicinal coupling constants J12,13axial =10.8 Hz, J14,13axial = 11.1 Hz and both of which were indicative of the anti axial-axial orientation of protons in C-12 and C-14 position with H-13axial and could be deduced that the orientation of H-12 and H-14 was parallel. The trans configuration of the C-12 and C-14 in exo-3a was assigned by the large vicinal coupling constants J12-13axial =12.3 Hz indicative of the axial orientation of H-12 and the significantly smaller vicinal coupling constants J14,13axial = 6.6 Hz typical for a gauche conformation of H-14 and H-13 and indicative of the equatorial orientation of H-14.
Table 1 Reaction of 4-hydroxy-3-phenyl[1,3]benzoxazin-2-one (1a-d) with olefins (2a-d)
Entry |
Substrates |
Time |
Product |
Yield |
|||||
R |
R1 |
R2 |
R3 |
R4 |
|||||
1 |
1a |
H |
H |
H |
H |
Ph |
30 |
3a |
63/30 |
2 |
1a |
H |
H |
H |
CH3 |
Ph |
30 |
3b |
70/25 |
3 |
1a |
H |
H |
CH3 |
H |
4-MeOC6H4 |
30 |
3c |
52/33 |
4 |
1a |
H |
H |
-CH2C6H4- |
H |
30 |
3d |
68/25 |
|
5 |
1b |
CH3 |
H |
H |
H |
Ph |
30 |
3e |
67b (74/26c) |
6 |
1b |
CH3 |
H |
H |
CH3 |
Ph |
30 |
3f |
62b(67/33c) |
7 |
1b |
CH3 |
H |
CH3 |
H |
4-MeOC6H4 |
30 |
3g |
53/28 |
8 |
1b |
CH3 |
H |
-CH2C6H4- |
H |
30 |
3h |
57/35 |
|
9 |
1c |
Br |
H |
H |
Ph |
CH3 |
30 |
31 |
62b (77/27c) |
10 |
1c |
Br |
H |
CH3 |
H |
4-MeOC6H4 |
30 |
3j |
58 /33 |
11 |
1c |
Br |
H |
- CH2C6H4- |
H |
30 |
3k |
68b (73/27c) |
|
12 |
1d |
(-CH=CH-)2 |
H |
H |
Ph |
30 |
3l |
56/37 |
|
13 |
1d |
(-CH=CH-)2 |
CH3 |
H |
4-MeOC6H4 |
30 |
3m |
46/32 |
|
14 |
1d |
(-CH=CH-)2 |
- CH2C6H4- |
H |
30 |
3n |
60/31 |
||
a: isolated yields of endo- and exo-
isomers;
b: only one isomer can be purified;
c: ratio determined by 1H NMR.
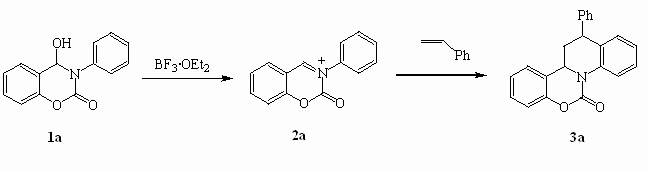
Scheme 2
3. EXPERIMENTAL
The starting materials 4-hydroxy-3-phenyl[1,3]benzoxazin-2-one (1a-c) and
4-hydroxy-3-phenyl[1,3]naphthoxazin-2-ones (1d) were prepared by the condensation
of 2-hydroxybanzaldehydes or 2-hydroxynaphthaldehydes with phenyl isocyanate in anhydrous
ethyl ether in the presence of triethylamine at room temperature, and purified by
recrystallization from anhydrous ethanol.
3.1 General Procedure for the Synthesis of 3a-k and 3l-n:
To a solution of the 4-hydroxy-3-phenylbenzoxazin-2-ones (1a-c) or the 4-hydroxy-3-phenylnaphthoxazin-2-one (1d) (2.0 mmol) and the olefin (2a-d) (2.5 mmol) in 50 mL anhydrous methylene dichloride was added at room temperature BF3·OEt2 (2.5 mmol) at portions under stirring. After continued stirring for half an hour, the reaction was quenched with an aqueous solution of sodium carbonate. The organic phase was washed with water and dried over anhydrous sodium sulfate. After the solvent was removed under reduced pressure the residue was separated on silica gel column eluted with chloroform and hexane (1:2 v/v) or by repeated preparative thin plate chromatography and recrystallized from chloroform and hexane (1:1 v/v) to give products as summarized in Table 1. All products were characterized.
3.2.1 endo-5,12,13,14-tetrahydro-14-phenylquino[1,2-c][1.3]benzoxazin-6-one (endo-3a) Mp 156-157 0C; 1H NMR (CDCl3) d 2.33 (t, 1H, J = 11.7 Hz, H-13ax), 2.63 (ddd, 1H, J = 11.7, 7.8, 2.4 Hz, H-13equ), 4.46 (dd, 1H, J = 11.4, 7.5 Hz, H-14), 4.92 (d, 1H, J = 12.8, H-12), 6.96 (d, 1H, J = 8.1 Hz, H-1), 7.06-7.12 (m, 2H), 7.14-7.19 (m, 3H), 7.20-7.33 (m, 6H), 7.73 (d, 1H, J = 8.1, H-4); 13C NMR (CDCl3) d 43.4 (C-13), 44.9 (C-14), 57.2 (C-12), 116.2 (C-4), 120.0 (C-11a), 124.5 (CH), 125.3 (CH), 125.4 (CH), 125.9 (CH), 126.5 (CH), 126.8 (CH), 128.3 (2C, C-2'), 128.8 (2C, C-3'), 129.3, 130.2, 132.5, 138.1 (C-4a), 144.7 (C-1'), 148.4 (C-7a), 149.2 (C=O); MS(EI) m/z (%) 327 (M+, 56), 312 (3), 298 (2), 248 (9), 236 (27), 208 (100), 165 (44). Anal. Calcd for C22H17NO: C, 80.71; H, 5.23; N 4.28. Found: C, 80.67; H, 5.28; N 4.22 %.
3.2.2 exo-5,12,13,14-tetrahydro-14-phenylquino[1,2-c][1.3]benzoxazin-6-one (exo-3a) dense oil; 1H NMR (CDCl3) d 2.37 (dd, 1H, J = 13.5, 2.4 Hz, H-13e), 2.64 (dt, 1H, J = 12.3, 2.4 Hz, H-13a), 4.42 (d, 1H, J = 6.6 Hz, H-12), 4.81 (dd, 1H, J = 12.3, 2.4 Hz, H-14), 6.94 (d, 1H, J = 7.2 Hz, H-1), 7.03-7.15 (m, 5H), 7.247-7.37 (m, 6H), 7.85 (d, 1H, J = 8.1, H-4); 13C NMR (CDCl3) d 39.9 (C-13), 41.7 (C-14), 52.1 (C-12), 116.1 (C-4), 120.8 (C-11a), 124.5 (CH), 124.8 (CH), 125 (CH), 125.4 (CH), 126.8 (CH), 126.9 (CH), 128.4 (2C, C-2'), 128.8 (2C, C-3'), 129.2, 130.6, 130.7, 135.5 (C-4a), 146.1 (C-1'), 149.4 (C=O); MS(EI) m/z (%) 327 (M+, 24), 312 (13), 298 (3), 249 (97), 236 (5), 208 (31), 91 (100). Anal. Calcd for C22H17NO: C, 80.71; H, 5.23; N 4.28. Found: C, 80.64; H, 5.32; N 4.23 %.
REFERENCES
[1] (a) Katritzky A R, Rachwal S, Rachwal
B. Tetrahedron, 1996, 52: 15031. (b) Kouznetsov V, Palma A, Ewert C et al. J. Heterocycl.
Chem., 1998, 35: 761. (c) Buonora P, Olsen J-C. Oh T. Tetrahedron, 2001, 57: 6099.
[2] Zhang W, Zheng A, Liu Z et al.. Tetrahedron Letters, 2005, 46: 5691.
[3] (a)
Waisser K, Bures O, Holy P et al. Pharmazie, 2003, 58: 83. (b) Waisser K, Kubicová L, Buchta V, et al. Folia Microbiol. (Praha), 2002, 47: 488. (c) Zhang P, Terefenko E. A, Fensome A, et al. J. Med. Chem, 2002, 45: 4379. (d )Takai H,
Obase H, Nakamizo N, et al. Chem. Pharm. Bull., 1985, 33: 1104.
[4] Crabb T. A, Mitchell J. S, J. Chem. Soc, Perkin
Trans. 2, 1977, 1592.
[5] Lempert K, Gyulai P. Tetrahedron, 1970, 26: 344.
通过N-苯基-N-酰亚胺正离子与烯烃的Diels-Alder反应合成喹啉并[1,2-c]
[1,3]苯并噁嗪-6-酮和喹啉并[1,2-c] [1,3]萘并噁嗪-6-酮
王俊蒲 黄利敏 张炜
(兰州大学化学化工学院,730000, 兰州)
摘要 4-羟基-3-苯基苯并噁嗪-2-酮 和4-羟基-3-苯基萘并噁嗪-2-酮在BF3
关键词 喹啉并[1,2-c] [1,3]苯并噁嗪-6-酮,喹啉并[1,2-c] [1,3]萘并噁嗪-6-酮,4-羟基-3-苯基苯并噁嗪-2-酮, Diels-Alder反应