http://www.chemistrymag.org/cji/2007/093013pe.htm |
Mar. 12,
2007 Vol.9 No.3 P.13 Copyright |
(College of Chemistry and Environmental Science, Hebei University, Baoding 071002; #Min Ke Environmental Detection of limited company, Baodong 071051) Received Jan. 24, 2007. Abstract 2- methyl - naphtho- (1, 2-d) thiazole is an important raw material for the synthesis of spectrum sensitization added dye in the photosensitive material industry. In order to enhance the purity and yield of this product, achieve the goal of reducing product cost, based on analyzing the production craft data, two intermediates in the synthesis process of this product are 1- acetyl amino naphthalene and 1- thioacetyl amino naphthalene. Their synthetic craft are instable, the data undulate in a big way. This article focused on two intermediates' synthesis crafts, its test result indicated that this method simplified and stabilized the synthetic craft, enhanced two intermediates' purity and yield, finally obtained the confirmation in the production.
Keywords 2- methyl - naphtho- (1, 2-d) thiazole; 1- acetyl amino naphthalene; 1- thioacetyl amino naphthalene; synthesis 1. INTRODUCTION
Since H.W.Vogel discovered that dye had spectrum sensitization-added function in 1873, in more than one century's time, the synthesis development and application of the spectrum sensitization added dye (mainly refers to Cyanine dye) have maken the important contribution to the photosensitive material industry [1]. Until now, the spectrum sensitization added dye was still one essential photography organic matter of kinds of new variety photosensitive material. Therefore, it continuously received the domestic and foreign film factory's regard to their synthesis and the application research.
Although the spectrum sensitization added dye had many types, it could be classified according to its structure and absorption wave length. According to the dye structure classification, it can be classified into cation dye, anion dye and neutral dye. For example, essence dye and styrene dye belong to cation dye.
Because in the molecular structure of cyanine, the chain length of the conjugated double bond was different, so the dye (340 ~ 1400nm) had different spectral absorption in the visible light to the near infrared light spectrum region, which had also formed the custom name of the spectrum sensitization added dye, there were feeling blue, feeling green, feeling red and feeling infrared spectrum and so on.
There were massive reports about the spectrum sensitization added dye's synthesis and application. We knew that 2- methyl - naphtho- (1, 2-d) thiazole was applied in the photosensitive material industry in order to enhance and improve color film feeling red rock's light sensitivity and color sensitivity. Recently, the massive synthetic research work had been made in the process of developing and producing this product.[2] But the yield still had 8 ~ 10% disparity compare to the overseas data. In order to enhance the yield and reduce the production cost, all data of this product were carefully analyzed. The intermediates of this product in first two steps are1- acetyl amino naphthalene and 1- thioacetyl amino naphthalene.Their synthesis craft was not stable, sulfuration step was particularly serious, so sulfuration's mass fluctuation was bigger. Therefore, this article emphasize on the improved synthesis of these two intermediates. 2. RESULT AND DISCUSSION
2.1 The synthetic route of 2- methyl - naphtho- (1, 2-d) thiazole
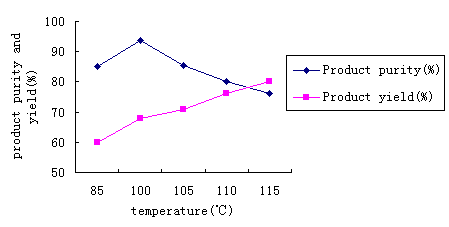
2.2.1 1- acetyl amino naphthalene's synthesis
In the original craft, after regurgitation, methyl-naphthele-amine and acetic anhydride were put into water with stirring, then they were centrifugated, laundered to neutrality and recrystallized from methanol or ethanol, finally the product was obtained, the purity was 93%, the yield was 85%. This craft had two problems: ①When 1- acetyl amino naphthalene was separated from water, it was easy to knot hard lump and hard to launder thoroughly and dry, it must be recrystallized from methanol or ethanol. Solvent used in crude product's recrystallization and the loss both increased the production cost. ②If crude product was directly smashed and used, the purity was 85%, it had a tremendous influence to the next step--sulfuration response. Using 50% acetic acid solution as medium, it could take acylation response at low temperature. When 1- acetyl amino naphthalene was separated from the acetic acid solution, the powder produced was easy to centrifugate, bath and dry could be obtained directly. Product purity was above 99%, the yield was 90%. Its shortcoming was increasing the glacial acetic acid amount and making the production cost increase.
2.2.2 1-thioacetyl amino naphthalene (sulfide for short in this article)
(1) Original synthetic craft and its problem
In the original craft, laminated phosphorus pentasulfide was used, 1- acetyl amino naphthalene and pyridine were separately joined to the reactor, heat up to 110 ~ 115 ℃ and reacted 45 minutes. After postprocessing there are many black sticky substances in the product, the purity was 80 ~ 85%, the yield was 73%, the result seriously affected the next step of oxidized closed loop response.
It had two problems: ①Using laminated phosphorus pentasulfide was a defect, because phosphorus pentasulfide assumed laminated shape, and it was easy to sink and pile up in the base. When the base was heat partially, it was easy to engender massive heat. If operation was improper, it was easy to make the material flush. ②At the high temperature 115 ℃, reacting 45 minutes was too long, massive outgrowth were produced, the sulfuration's color was heavier, the product purity was lower(usually 80 ~ 85%), the yield was 73%.
(2) Improved synthetic craft
① Changing phosphorus pentasulfide granularity
Because 1- acetyl amino naphthalene was powdery, so it was evenly mixed with smashing phosphorus pentasulfide and put into the reactor containing pyridine, in order to let two reactants (1- acetyl amino naphthalene and phosphorus pentasulfide) increase the contact face between them in pyridine.
The test result indicated that the entire experiment was easy to control, but because feeding raw material needed a long time(usually 1 ~ 2 hours), so the final result wasn't ideal, there were many black matters, the product purity and yield hadn't anticipated requirement.
② Changing the way of feeding material
1- acetyl amino naphthalene and phosphorus pentasulfide were mixed and added to the reactor, and then pyridine was added, finally stiring was started.
In the entire process, the reaction was steady and easy to control. The test result indicated that optimal reaction condition was this: reaction temperature was 100 ~ 105 °C, the reaction time was 15 ~ 30 minutes , the product purity and yield enhanced.
When pyridine was joined and stiring was started, the reactant automatically elevated temperature to 60°C.When the reactant was heat up to 80 ~ 85 °C and added to pyridine in the fluid, the fluid changed thin and was easy to stir evenly.When the temperature was elevated to 95 ~ 100 °C, the reaction material fluid's color changed as this rule: light yellow -> red -> garnet.When the temperature was at 100 °C, the response fluid gradually stiffened and formed the scarlet red response fluid.
③ Seeking the best reaction temperature and time match to experiment
According to the above test result, at the situation of unchanging present material ratio, the best reaction temperature and time were sought to achieve the goal of enhancing sulfuration's purity and yield.
A series of experiments were performed under different temperatures, and the reaction time was 45min. The results are given in Table 2 and Figure 1.
Table 2 temperature
's affectionTemperature(℃) |
85 |
100 |
105 |
110 |
115 |
Product purity(%) |
85 |
93.8 |
85.5 |
80 |
76 |
Product yield(%) |
60 |
68 |
70.7 |
76.3 |
80 |
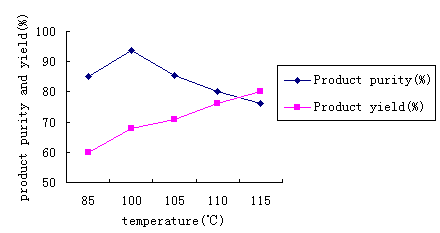
Fig.1 Temperature's affection
As can be seen from Fig.1, the purity was enhanced along with the temperature was
increased. when the temperature was 100°C, the yield was 94%
and slowly dropped to 76%. The yield unceasingly enhanced along with temperature
increased, but the product purity dropped. This indicated that the purity was higher and
the reaction effect was better when reaction temperature was 100 °C.
A series of experiments were
performed to search the best reaction time, because the reaction time had more tremendous
influence on the extent of reaction. When the reaction temperature was 100°C and
other conditions were unaltered too(Only the reaction time was changed), the results are
shown in Table 3 and Figure 2. The product color was deeper and deeper when the reaction
time prolonged, the product purity increased incipiently and reducing gradually, and its
yield slightly dropped.
Table 3 reaction time
's affectionReaction time(min) |
15 |
20 |
30 |
40 |
45 |
50 |
60 |
Product purity(%) |
85 |
88 |
93.9 |
90 |
88 |
86 |
80 |
Product yield(%) |
70 |
73 |
80 |
78.6 |
72 |
70 |
68 |
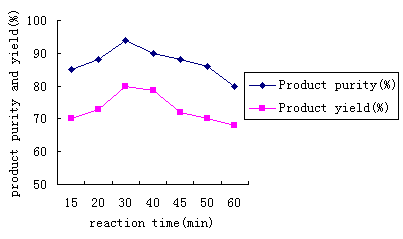
As can be seen from Fig.2, the product purity changed lower again when the reaction time was longer than 30min, there was one peak(the product purity is 93.9%) when the reaction time was 30 min. The product yield rose from 70% to 80% and then slowly reduced to 68%. Two curves' trends were similar, the yield and purity was most ideal when the reaction time was 30 min.
The experimental results indicate that when the reaction time was 30 min and the reaction temperature was 100°C, the product purity and yield was the ideal result. Four repeated tests' results were given in Table 4. As can be seen from Table 4, the reaction condition--the reaction temperature and the reaction time were best matched.
Table 4 The results of four tests
number |
1 |
2 |
3 |
4 |
purity(%) |
93.5 |
94 |
94 |
95 |
Yield(%) |
79.5 |
80 |
81 |
80.5 |
(3) Terminating the sulfuration
There were many deficiencies in sulfuration
and post-processing in original craft. A
It took a long time that using the 8%NaOH solution to dissolve coagulate sulfuration under the room temperature. (Under the high temperature, dissolving sulfuration with the alkali fluid induced the sulfuration easy to decompose and the yield was lower.) So such operation had the shortcoming that the produce cycle was long and product purity was low.
In order to make improvement to the post-processing craft, the following improvement was made to the sulfuration post-processing operation:After sulfuration reaction finishing, the high temperature fluid was immediately put in the quota ice water (prepared in advance, ice was less than water). Sulfuration formed one softer brown thing (The temperature was below 20 °C now).The 40% NaOH solution was added, sulfuration coagulation dissolved quickly under stirring and turned to be transparent red solution (temperature was below 35 °C now).After calculating, the total volume of such solution was the same to the original formula. A few insoluble matter were wiped off, and acid was dropped to let the sulfuration separate out. After that operation, not only the same effect was achieved, but also the production cycle was reduced greatly.
2.3 Experimental part
2.3.1 Acylation synthesis
alpha-naphtyylamines (100 g, 0.69 mol) was dissolved in the acetic acid (50%, 2750 mL), after dissolving completely, one time water was supplied, the temperature was heat up to 50°C, the inner temperature was controled at 50 ~ 60°C, the acetic anhydride (100 g, 0.8 mol) was added dropwise, and the reaction mixture was stirred for 1 h at 60 °C.The product was cooled to the room temperature, after the mother liquor was pulled out, the product was washed to neutral with water. The product was dried to 116 g (90 % yield), the purity was 99.8%.
2.3.2 Sulfuration synthesis
Thiophosphoric anhydride (24 g) was smashed and mixed with acylation reagent (40 g) evenly. The solid raw material was completely added to the reactor first, and then the pyridine (34 ml) was added. In the reactant heat rose to 76 ~ 80°C, temperature was elevated to 100°C, reacted for 30 min at 100°C.The reactant was put into the ice water (260 mL). It was stirred for 20 min to become dispersed shape, and then it was added to 40% NaOH solution, cooled to the room temperature, dissolved completely and filtered.The filtrate was cooled to 0 ~ 5°C, 3% acetic acid solution was added dropwise in 2 ~ 3 h, ph=6 ~ 7.The mother liquor was pulled out and washed three times with 0.5% vinegar acid, the product was pulled out, and the light brown powder was obtained. Dry product: 34 ~ 36 grams, purity: 93 ~ 95%, yield: 77 ~ 80%, melting point: 97~97.5°C.
Fig .3 2- methyl - naphtho- (1, 2-d) thiazole's HPLC Spectrum
3. CONCLUSION
The two intermediates' improved synthetic crafts were improved and applicable in
the production process. Comparison between the above experiment and the earlier result
indicate that the yield and purity of product have enhancement.The yield of 1 - acetyl
amino naphthalene enhanced from 85% to 90 ~ 92%, and its purity increased from 93% to 99.7
~ 99.9%.The yield of 1 - sulfo- acetyl amino naphthalene increased from 73.9%
to 77 ~ 80%, and its purity rose from 80 ~ 85% to 93 ~ 95%.The practice proved that, fast
terminating sulfuration reaction end point reduced sulfuration production cycle greatly
compared with the old craft, the effect was remarkable.
REFERENCES
[1] Rongqi Chen, The development and application of green dye, Printing and Dyeing,2006,(6): 45.
[2] Hamer F M. The cyanine dyes and related compound [M]. New York: Interscience
Publishers Inc., 1964(198)
[3] Morita, Kosuke, Ebara, Kazuya, New positive-type photosensitive polyimide having sulfo
groups,Polymer,
2003,(44):20
[4] Robert E. Buckle M. Peter B. Organic Compound Synthesis [M].Vol.IV. New York: John
Wiley&Sons Inc., 1963,722.
2-甲基-萘并(1,2-d)噻唑的合成改进研究
李占臣,赵爱琴#,韩雪,董志民#,窦兰凤#
(河北大学化学与环境科学学院, 保定071002; #河北省保定市民科环境检测有限公司,071051)
摘要 2-甲基-萘并(1,2-d)噻唑是感光材料工业中合成光谱增感染料的重要原材料之一,为了提高该产品的纯度和收率,达到降低生产成本的目的,在分析该产品的生产工艺数据的基础上,认为合成该产品过程中的两个中间体:1-乙酰氨基萘和1-硫代乙酰氨基萘的合成工艺不稳定,数据波动大,尤其是后者更为严重。本文就两个中间体的合成工艺进行了讨论和试验,其试验结果表明:简化和稳定了合成工艺,提高了两个中间体的纯度和收率,并最终在生产中得到验证。
关键词 2-甲基-萘并(1,2-d)噻唑,1-乙酰氨基萘,1-硫代乙酰氨基萘,合成