http://www.chemistrymag.org/cji/2008/101002pe.htm |
Jan. 1,
2008 Vol.10 No.1 P.2 Copyright |
(1 Department of Chemistry, Jinan University , Guangzhou 510632, China; 2 Department of Chemistry and Life Science, West Anhui University, Lu,an 237012, China) Abstract The novel ZnS hollow submicrospheres with uniform open holes and multiple open holes were prepared by reacting ZnSO4 with sulfide source from CS2 and ethylenediamine at 50oC, then placing the mixture at room temperature. The ZnS hollow submicrospheres were characterized by XRD, SEM, TEM and UV-vis absorption. As the placing time of the reaction mixture increased from 2 days to 5 days, the size of ZnS hollow submicrospheres with holes decreased from about 700-750 nm to 150-200 nm. A rational mechanism is proposed that the spherical shape is formed by ZnS nanocrystals aggregating around the product of ethylenediamine and CS2, open holes are caused by evaporation of CS2 in the drying process, and hollow submicrospheres with smaller size are obtained by nanocrystals reaggregating with the aid of product of ethylenediamine and CS2.
Keywords: zinc sulfide; submicrosphere; ethylenediamine; open hole
1. INTRODUCTION
As template and sulfur source, ethylenediamine was employed in the preparation of hollow spheres of CdS[9]. But no such report about ZnS has been found. The special role of ethylenediamine aroused our much interesting. In order to further investigate the action of ethylenediamine and its product with CS2, reaction of ZnSO4 with sulfur source from CS2 and ethylenediamine was carried out at 50oC. The effects of placing time on size of ZnS and mechanism of crystal growth of ZnS were discussed also. 2. EXPERIMENTAL
2.1. Synthesis
All chemicals were of analytical purity and used without further purifications. All of the solutions were prepared with twice distilled water. In a typical synthesis, 0.37 mL of CS2 was added into 40 mL of 0.15 mol/L ethylenediamine aqueous solution in a flask. Then 20 mL of 0.1 mol/L ZnSO4 aqueous solution was added. The mixture was heated to 50oC and kept at this temperature for 20 min. Then, the mixture was cooled and maintained under a static condition for a definite time at room temperature. The white precipitate was centrifuged and washed with water and ethanol for three times, respectively. The final powder product was dried at 60oC in a vacuum oven for 6 h.
2.2. Characterization
The products were subject to the characterization of X-ray diffraction (XRD, MSAL XD-2 powder X-ray diffractometer using Cu Ka radiation of wavelength 1.5406Å at 40 kV and 20 mA), scanning electron microscopy (SEM, JEOL JSM-T300), transmission electron microscopy (TEM, Philips, Tecnai-10, 100 kV), UV-visible adsorption spectroscopy (UV-vis, type TU-191, Beijing).
3. RESULTS AND DISCUSSION
3.1 Effect of placing time on the size of ZnS hollow submicrospheres with open holes
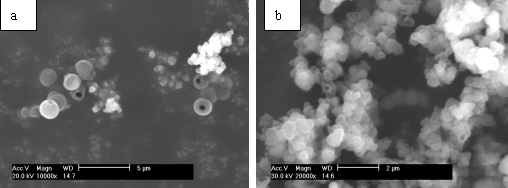

Fig. 1. SEM images of ZnS hollow submicroscpheres by placing the reaction mixture for a) 2 days, b) 3 days, c) 5 days
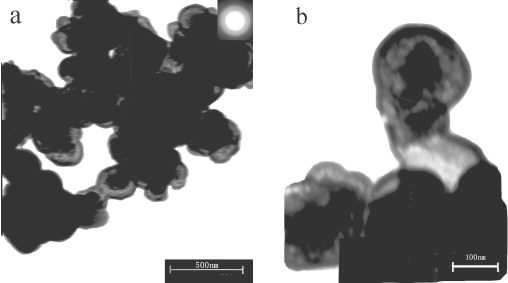
Fig. 2. TEM images of ZnS hollow submicroscpheres by placing the reaction mixture for 5 days
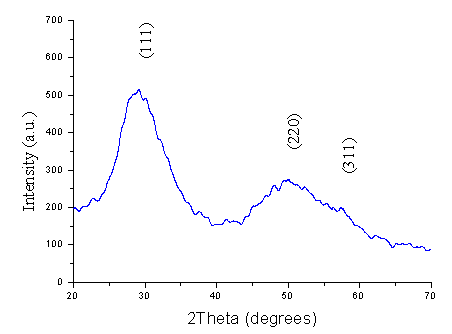
Fig. 3. X-ray diffraction pattern of ZnS hollow submicrosphere
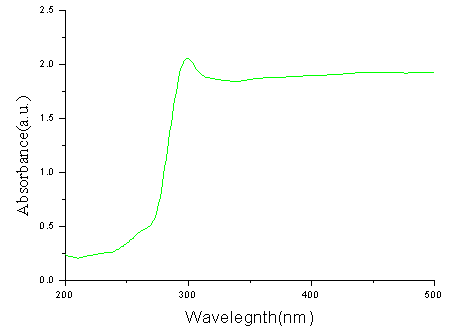
Fig. 4. UV-vis absorption spectrum of ZnS hollow submicroscphere Different size ZnS particles obtained at different setting time exhibit similar X-ray diffraction and UV-vis absorption. This result confirms they are consisted of nanoparticles again. Fig. 3 shows the XRD pattern of ZnS hollow submicrospheres. The three broad characteristic diffraction peaks appear at about 28.5°, 47.5°and 56.3°, corresponding to the (111), (220), and (311) planes of the zinc blende, the cubic ZnS phase. The average crystallite size is estimated to be about 4 nm according to the line width analysis of the (111) diffraction peak based on the Scherrer formula, suggesting that the submicrospheres with open holes could be consisted of primary nanoparticles, which is in good agreement with the enlarged TEM.
Fig. 4 presents the UV-vis absorption spectrum of the ZnS hollow submicrospheres, which were obtained after the dried product was redispersed in ethanol. It shows an absorption peak at 294 nm, which is obviously blue-shifted from 340 nm for bulk zinc blende ZnS due to quantum size effects.
It has been documented that the optical absorption of the ZnS nanocrystals with a size of 3.5 nm exhibits an excitonic peak at 288 nm [10], the size of crystallite constituting ZnS hollow submicrspheres could be estimated to be 3.8 nm according to the excitonic peak at 294nm, which is in good agreement with the result obtained from XRD.
3.2 Possible mechanism
CS2 and ethylenediamine were employed as the sulfur source for ZnS. The synthetic design was motivated by the known reactions between CS2 and ethylenediamine[11], as described by the following equations:
H2N-CH2-CH2-NH2+CS2→H2N-CH2-CH2-NH-CS-SH (1)
n(H2N-CH2-CH2-NH-CS-SH) →(-HN-CH2-CH2-NH-CS-)n + H2S (2)
It is well known that ethylenediamine is a bidentate ligand and can react with metal ions to form relatively stable complexes, which may serve as molecular templates in control of the crystal growth[12]. Thus, Zn2+ could be chelated by ethylenediamine in aqueous solution, and carried from water to CS2-water interface[9,13]. Then ZnS nanocrystals would be formed by reacting Zn2+ with H2S produced immediately after the contact of ethylenediamine with CS2. The amino groups in the product in reaction (2) can still act as ligands with ZnS, and would attract ZnS to precipitate around the organic phase. In aqueous solution, the hydrophobility of organic phase drive the organic molecule to form microscale spherical oil droplet, which resulting in the spherical ZnS particles precipitated around the organic core containing the product in reaction (2) and CS2. The interesting uniform open holes in present ZnS submicrospheress are probably caused by the evaporation of CS2 in the drying process, which is proposed to result in the close hollow CdS spheres[9]. This is supported by the ZnS submicrospheres with novel multiple holes obtained by employing higher ratio CS2 to ethylenediamine as shown in Fig. 5a and b. The more amount of CS2 in the core cause more holes in ZnS submicrospheres.
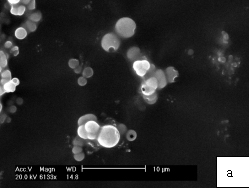

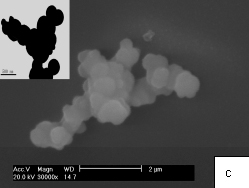
Fig. 5 SEM images of ZnS hollow submicroscpheres with different molar ratio of CS2 : ethylenediamine by setting the reaction mixture for 2 days: (a) 3:1, (b) 5:1, (c) 1:3, c), inside corresponding TEM images,Scale bars: 500nm
Ethylenediamine has been employed to form complex precursor of ZnS and probably acts as connecting molecular bridges between neighboring ZnS chains or layers
[14]. In the synthesis of binary transition metal sulfides nanocrystallites MS (M = Zn, Cd, Co, Ni) with hydrothermal method, ethylenediamine is the key factor to the purity and morphology of the products[15]. The controlled fabrication of wurtzite ZnS nanorods is due to a mediated generation of the lamellar phase, ZnS·0.5ethylenediamine, a covalent organic-inorganic network based on ZnS slabs, and to its subsequent thermolysis in aqueous solution [16]. Thus, ethylenediamine can act not only with Zn2+ ions but also with ZnS molecules. For this reason, the product with similar structure of ethylenediamine unit in reaction (2) is suggested to template the ZnS nanocrystals reaggregate from the larger one during setting. Thus, the same formation process as initial hollow ZnS submicrospheres with open holes is repeated to give the corresponding smaller ZnS particles. The template effect of ethylenediamine and its product can be supported by disappearance of hole after adding surfactants such as SDS, SDBS. Due to the well known strong action of template, surfactants would induce the formation of ZnS particles instead of ethylenediamine and its product. Thus the forming process proposed above is replaced by the role of surfactants also, which causes the disappearance of special morphology correspondingly. Further support comes from the smaller ZnS particles obtained not by long setting time, but by employing more amount of ethylenediamine and setting for only 2 days as shown in Fig. 5c. The SEM and related inside TEM show no hole in the ZnS particles. In the case of excess ethylenediamine, almost all CS2 have been consumed, and the left ethylenediamine would induce the formation of ZnS nanocrystals instead of its product with CS2 for its lower molecular weight and stronger interaction with Zn2+. Furthermore, the good solubility in water of ethylenediamine would not put the molecules exist as sphere. Upon these reasons, small size ZnS particles without uniform spherical shape and hole are resulted in.4. CONCLUSIONS
The novel ZnS hollow submicrospheres with uniform open holes and multiple open holes
were prepared by reacting ZnSO4 with sulfide source from CS2 and
ethylenediamine at 50℃, then placing the mixture at
room temperature. With increasing the setting time of the reaction mixture, the ZnS
submicrospheres became smaller. A rational mechanism is proposed that the spherical shape
is formed by ZnS nanocrystals aggregating around the product of ethylenediamine and CS2,
open holes are caused by evaporation of CS2 in the drying process, and hollow
submicrospheres with smaller size are obtained by nanocrystals reaggregating with the aid
of product of ethylenediamine and CS2.
[1] Scott R W J, MacLachlan M J, Ozin G A, Curr. Opin. Solid State Mater. Sci. 4 (1999) 113.
[2] Yin H, Wada Y, Kitamura T, et al. Environ. Sci. Technol. 35 (2001) 227.
[3] Brn P L , Holmes A B, Brown D D C, et al. Nature (London) 356 (1992) 47.
[4] Loukanov A R, Dushkin C D, Papazova K I, et al. Colloids Surf. A 245 (2004) 9.
[5] Svechnikov S V, Zavyalova L V, Roshchina N N, et al. Semiconductors 34 (2000) 1128.
[6] He Y J, Mater. Res. Bull. 40 (2005) 629.
[7] Ma Y R, Qi L M, Ma J M, et al. Langmuir 19 (9) (2003) 4040.
[8] Zhao Y B, Chen T T, Zou J H, et al. J. Cryst. Growth 275 (2005) 521.
[9] Huang J X, Xie Y, Li B, et al. Adv. Mater. 12 (2000) 808.
[10] Nanda J, Sapra S, Sarma D D, Chem. Mater. 12 (2000) 1018.
[11] Chen N L, Handbook of Solvents, second ed., Chemical Industry Press, Beijing, 1994 (Chinese).
[12] Li B, Xie Y, Huang J X, et al. Adv. Mater. 11 (1999) 1456.
[13] Fu X, Wang D B, Wang J, et al. Mater. Res. Bull. 39 (2004) 1869.
[14] Deng Z X, Wang C, Sun X M, et al. Inorg. Chem. 41 (4) (2002) 869.
[15] Wang Q S, Xu Z D, Yin H Y, et al. Mater. Chem. Phys. 90 (2005) 73.
[16] Chen X J, Xu H F, Xu N S, et al. Inorg. Chem. 42 (2003) 3100.
在乙二胺和CS2溶液中简便合成带有开口孔的中空ZnS亚微球
李林刚1,2,唐渝1,杨骏1,张渊明1,杜碧莹1
(1暨南大学化学系,广东 广州510632;2皖西学院化学与生命科学系,安徽 六安 237012)
摘要 在50℃通过ZnSO4与CS2与乙二胺(en)产生的硫源反应,然后于室温下放置合成了具有均一开口孔和多孔新形貌的中空ZnS亚微球。对合成的ZnS亚微球进行了XRD, SEM, TEM 以及 UV-vis等测试手段的表征,当放置时间由2天增加到4天,带孔的中空ZnS亚微球的大小由700-750 nm 减小到 150-200 nm。提出了合理的生长机理,认为由ZnS纳米晶在en与CS2形成的带有氨基的产物周围聚集形成了球形,CS2在干燥过程中蒸发形成了开口孔,在带氨基产物的协助下纳米晶再聚集形成了尺寸变小的中空亚微球。
关键词 硫化锌;亚微球;乙二胺;开口孔