http://www.chemistrymag.org/cji/2008/105023pe.htm
May 1,
2008 Vol.10 No.5 P.23 Copyright ![]()
http://www.chemistrymag.org/cji/2008/105023pe.htm |
May 1,
2008 Vol.10 No.5 P.23 Copyright |
Liquid-liquid equilibria of quaternary system with two partially miscible solvent pairs: water + methanol + 1,1-dimethylethyl methyl ether + benzene
Chen Yao, Cao Chenyu, Fu Min, Chen Enping, Zhang Yansong1. INTRODUCTION
1,1-Dimethylethyl methyl ether(MTBE),
1,1-dimethylpropyl methyl ether(TAME), and diisopropyl ether(DIPE) are commonly added into
gasoline to improve the octane rating and reduce the air-pollution. In the recent years,
studies of phase equilibria of multicomponent systems consisting of oxygenated compounds,
such as MTBE, TAME and DIPE, have gained interest as can be seen in literatures [1-5].
The thermodynamic behavior of these components in liquid mixtures is also of interest not
only in its application for reformulated gasoline but also in extraction or separation
operations with other components.
In this work, we report experimental liquid-liquid equilibrium(LLE)
data for quaternary mixtures of (water + methanol + MTBE + benzene), and relevant ternary
mixtures of (water + MTBE + benzene) at 25°C. The experimental results were correlated by
means of the extended and modified UNIQUAC models [6,7] including both ternary
and quaternary parameters coming from multicomponent intermolecular interactions, in
addition to binary parameters. The binary vapour-liquid equilibria(VLE), mutual solubility
and ternary LLE relevant to the quaternaries have been available from the literatures:
(methanol + benzene) [8], (methanol + MTBE) [9], (methanol + water) [10],
(MTBE + benzene) [11], (water + benzene) [12], (water + MTBE) [13],
(water + methanol + benzene) [14], (water + methanol + MTBE) [15],
and (water + MTBE + benzene) measured in this work.
2. EXPERIMENTS
2.1 Materials
MTBE was supplied by Tedia Company, Inc. with minimum mass fraction purity of 0.998.
Methanol and benzene were provided from Guangzhou Chemical Reagent Factory, with mass
fraction purities of 0.995 and 0.997, respectively. Water provided from Jinan University
was distilled twice and had a mass fraction purity of 0.999. The g.c. analysis gave mass
fractions purities of 0.997 for MTBE, 0.995 for benzene, and 0.998 for methanol. All
chemicals were used directly in this work.
2.2 Apparatus and Procedures
Ternary and quaternary LLE
measurements were carried out at the temperature (298.15 ± 0.01) K. The experimental
apparatus was the same that reported in detail previously [4]. The quaternary
mixtures whose volume is about 80 cm3 were loaded in the equilibrium glass cell
placed in a thermostated water bath. The mixture was then stirred vigorously by magnetic
stirrer for 3 h and allowed to settle 3 h, which was sufficient for separation into two
phases. Dry nitrogen gas was used to prevent contamination with moisture in the headspace
of the equilibrium cell. The liquid samples about 5 cm3, withdrawn from both
upper and lower phases in the cell by using a milliliter syringe without changing the
phase equilibria between two layers, were analyzed by a gas chromatograph (Shimadzu,
GC-14C) equipped with a thermal conductivity detector. Each component of the ternary and
quaternary mixtures was separated clearly using a stainless steel column (2 m long, 3 mm
i.d.) packed with Porapak SQ. The temperatures of the injection and detector were set at
483.15 K. The initial temperature and final temperature of the oven were kept at 453.15 K.
The hydrogen flow rates for both the separation and reference columns were set at 1.0 cm3· s―1.
The peak areas of the components, detected with a chromatopac (Zhejiang, N2000), were
calibrated by gravimetrically weighted mixtures. The mass of each component of the mixture
was determined from the calibration and converted to mole fraction. Three analyses at
least for each sample were made to obtain a mean value. The accuracy of the experimental
measurements was estimated to be within ±0.001.
The quaternary mixtures for (water + methanol + MTBE + benzene) were
prepared by mixing binary mixtures of (MTBE + benzene) whose compositions are M1, M2, and
M3 with water then methanol stepwise to cover the two-phase region shown in Figure 1.
Figure 1 shows schematically a tetrahedron to depict three planes of the quaternary
mixtures of (water + methanol + MTBE + benzene). The values of M1, M2, and M3 are
approximate 0.25, 0.50, and 0.75, respectively, indicating the mole fraction of MTBE in
(MTBE + benzene).
2.3 Experimental results
Tables 1 and 2 show experimental LLE data for the (water + MTBE + benzene) and (water +
methanol + MTBE + benzene) mixtures.
Table 1 Equilibrium phase compositions in mole fraction (x) for the ternary of (water + MTBE + benzene) at 25
ºCorganic phase |
aqueous phase |
||||
x1 |
x2 |
1-x1-x2 |
x1 |
x2 |
1-x1-x2 |
0.0379 |
0.6955 |
0.2666 |
0.9944 |
0.0056 |
0.0000 |
0.0298 |
0.5712 |
0.3990 |
0.9955 |
0.0045 |
0.0000 |
0.0250 |
0.4834 |
0.4916 |
0.9959 |
0.0039 |
0.0002 |
0.0215 |
0.4228 |
0.5557 |
0.9965 |
0.0032 |
0.0003 |
0.0213 |
0.3675 |
0.6112 |
0.9966 |
0.0030 |
0.0004 |
0.0176 |
0.3333 |
0.6491 |
0.9971 |
0.0026 |
0.0003 |
0.0152 |
0.2919 |
0.6929 |
0.9975 |
0.0022 |
0.0003 |
0.0202 |
0.2359 |
0.7439 |
0.9979 |
0.0021 |
0.0000 |
0.0412 |
0.7819 |
0.1769 |
0.9936 |
0.0064 |
0.0000 |
0.0410 |
0.6248 |
0.3342 |
0.9953 |
0.0047 |
0.0000 |
0.0267 |
0.5198 |
0.4535 |
0.9963 |
0.0037 |
0.0000 |
Table 2 Equilibrium phase compositions in mole fraction
x for the quaternary mixtures of (water + methanol + MTBE + benzene) at 25ºCorganic phase |
aqueous phase |
|||||
x1 |
x2 |
x3 |
x1 |
x2 |
x3 |
|
{ x1water +x2methanol + x3MTBE+(1-x1-x2-x3)benzene} |
||||||
M1= 0.25 |
||||||
0.0213 |
0.0222 |
0.2932 |
0.9042 |
0.0924 |
0.0034 |
|
0.0271 |
0.0476 |
0.2686 |
0.8267 |
0.1694 |
0.0039 |
|
0.0400 |
0.0777 |
0.2490 |
0.7508 |
0.2434 |
0.0058 |
|
0.0418 |
0.0835 |
0.2024 |
0.7105 |
0.2808 |
0.0067 |
|
0.0463 |
0.1012 |
0.1939 |
0.6717 |
0.3173 |
0.0081 |
|
0.0471 |
0.1301 |
0.1881 |
0.6109 |
0.3735 |
0.0111 |
|
0.0500 |
0.1420 |
0.1554 |
0.5552 |
0.4210 |
0.0132 |
|
0.0456 |
0.1516 |
0.1453 |
0.5429 |
0.4326 |
0.0131 |
|
0.0457 |
0.1674 |
0.1331 |
0.4837 |
0.4770 |
0.0172 |
|
0.0503 |
0.1826 |
0.1225 |
0.4489 |
0.5037 |
0.0197 |
|
0.0297 |
0.0600 |
0.1992 |
0.7623 |
0.2330 |
0.0047 |
|
0.0408 |
0.1045 |
0.1739 |
0.6411 |
0.3453 |
0.0090 |
|
M2= 0.50 |
||||||
0.0600 |
0.1067 |
0.3906 |
0.7631 |
0.2275 |
0.0094 |
|
0.0959 |
0.1788 |
0.3570 |
0.6799 |
0.2996 |
0.0177 |
|
0.1117 |
0.2295 |
0.3147 |
0.6126 |
0.3561 |
0.0257 |
|
0.1249 |
0.2561 |
0.2946 |
0.5814 |
0.3793 |
0.0311 |
|
0.1179 |
0.2451 |
0.2904 |
0.5882 |
0.3764 |
0.0281 |
|
0.0561 |
0.0925 |
0.4655 |
0.8046 |
0.1864 |
0.0090 |
|
0.0321 |
0.0184 |
0.4929 |
0.9388 |
0.0563 |
0.0049 |
|
0.0464 |
0.0590 |
0.4535 |
0.8620 |
0.1315 |
0.0065 |
|
0.0884 |
0.1548 |
0.3633 |
0.6997 |
0.2831 |
0.0152 |
|
0.1005 |
0.2001 |
0.3270 |
0.6399 |
0.3334 |
0.0219 |
|
M3= 0.75 |
||||||
0.0768 |
0.0516 |
0.6489 |
0.8898 |
0.1017 |
0.0085 |
|
0.0982 |
0.1013 |
0.5893 |
0.8232 |
0.1655 |
0.0113 |
|
0.1424 |
0.1781 |
0.4972 |
0.7360 |
0.2439 |
0.0201 |
|
0.1605 |
0.2131 |
0.4549 |
0.7074 |
0.2680 |
0.0231 |
|
0.1820 |
0.2488 |
0.4090 |
0.6699 |
0.2949 |
0.0323 |
|
0.2387 |
0.3046 |
0.3210 |
0.5996 |
0.3421 |
0.0507 |
|
0.2126 |
0.2884 |
0.3436 |
0.6056 |
0.3396 |
0.0473 |
|
0.2126 |
0.2995 |
0.3254 |
0.5975 |
0.3449 |
0.0485 |
|
0.0730 |
0.0421 |
0.6569 |
0.9036 |
0.0887 |
0.0077 |
|
0.0931 |
0.0866 |
0.6051 |
0.8384 |
0.1515 |
0.0101 |
|
0.1129 |
0.1434 |
0.5468 |
0.7798 |
0.2053 |
0.0149 |
|
0.1909 |
0.2586 |
0.3911 |
0.6487 |
0.3101 |
0.0372 |
|
3. CALCULATION PROCEDURE AND RESULTS
3.1 Calculation procedure
The extended UNIQUAC [6] and modified UNIQUAC [7] models including binary and multibody interaction
parameters were used to correlate the experimental LLE data.
The binary parameter![]() defined by the binary energy parameter aji
is expressed as
defined by the binary energy parameter aji
is expressed as
![]() (1)
(1)
The binary energy parameters for the miscible mixtures were obtained
from the VLE data reduction using the following thermodynamic equations [16]:
![]() (2)
(2)
![]() (3)
(3)
where P, x, y, and g are the total
pressure, the liquid-phase mole fraction, the vapor-phase mole fraction, and the activity
coefficient, respectively. The pure component vapor pressure, ![]() , was calculated by using
the Antoine equation with coefficients taken from the literatures [17,18]. The
liquid molar volume,
, was calculated by using
the Antoine equation with coefficients taken from the literatures [17,18]. The
liquid molar volume, ![]() ,
was obtained by a modified Rackett equation [19]. The fugacity coefficient, F, was calculated
by the eqn.(3). The pure and cross second virial coefficients, B, were estimated by
the method of Hayden and O'Connell [20]. The binary energy parameters for the
partially miscible mixtures were obtained by solving the following thermodynamic equations
simultaneously.
,
was obtained by a modified Rackett equation [19]. The fugacity coefficient, F, was calculated
by the eqn.(3). The pure and cross second virial coefficients, B, were estimated by
the method of Hayden and O'Connell [20]. The binary energy parameters for the
partially miscible mixtures were obtained by solving the following thermodynamic equations
simultaneously.
![]() (4)
(4)
![]() and
and ![]() ( I, II = two
liquid phases ) (5)
( I, II = two
liquid phases ) (5)
Ternary parameters t231, t312, and t123 were obtained by fitting the two models to
the ternary LLE data and then the quaternary parameters t2341, t1342, t1243 and t1234 were
determined from the quaternary experimental LLE data using a simplex method [21]
by minimizing the objective function:
F=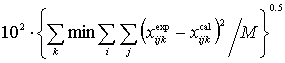 (6)
(6)
where min denotes minimum values, i = 1 to 3 for ternary mixtures or i = 1
to 4 for quaternary mixtures, j = 1, 2 (phases), k = 1, 2, ...,M (number of tie lines), M = 2ni, and x is the
liquid-phase mole fraction.
3.2 Calculation results
Table 3 shows the molecular-structural volume and area parameters, r and q,
for MTBE taken from the reference [18], while the others are taken from
Prausnitz et al. [16], together with the interaction correction factor q',
for which the value for self-associating components was taken from the literature [6,7],
while that for nonassociating components was set to q' = q0.20 in the extended UNIQUAC model and q'
= q0.75 in the modified UNIQUAC model.
Table 4 presents the constituent binary energy parameters of the modified and extended
UNIQUAC models. Table 5 shows the ternary parameters obtained in fitting the modified and
extended UNIQUAC models to the experimental ternary LLE systems, and root-mean-square
deviation of the mole fraction of tie lines between the experimental and calculated
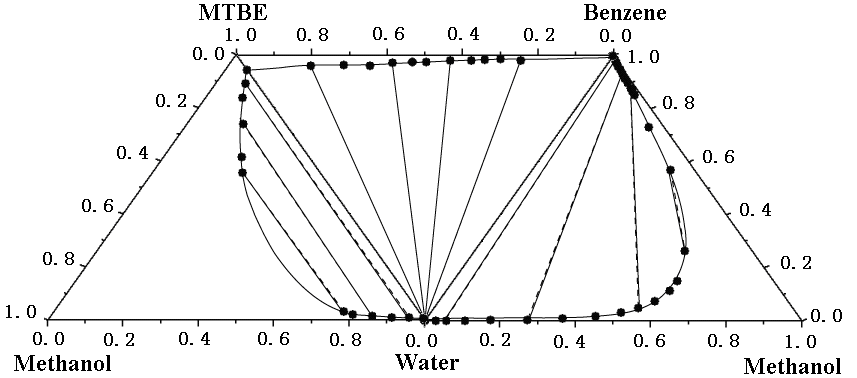
results for the systems. Figure 2 compares the experimental and correlated LLE of the
ternary mixtures making up the quaternary mixtures of (water + methanol + MTBE + benzene)
at 25ºC. The quaternary system exhibits type
2 quaternary LLE behavior, which is composed of two ternary LLE for the (water + methanol
+ MTBE) and (water + methanol + benzene) are classified as type 1, and one ternary LLE for
the (water + MTBE + benzene) as type 2 are illustrated in Figure 2. Table 6 summarizes
the correlated results for the quaternary mixtures obtained in fitting the extended and
modified UNIQUAC models with binary, ternary, and quaternary parameters to the
experimental quaternary LLE data, together with the predicted results by these models with
only binary parameters listed in Table 4. It seems that the extended UNIQUAC model
correlated the quaternary LLEs more successfully than the modified UNIQUAC model, and both
the models can give a much more accurate representation for the quaternary LLEs by
including the ternary and quaternary parameters in addition to the binary ones.
Table 3 Structural parameters for pure components
Component |
r |
q |
q'a |
q'b |
water |
0.92 |
1.40 |
1.28 |
0.96 |
methanol |
1.43 |
1.43 |
1.48 |
1.00 |
benzene |
3.19 |
2.40 |
q 0.75 |
q 0.20 |
MTBE |
4.07 |
3.63 |
q 0.75 |
q 0.20 |
a
Modified UNIQUAC model.Table 4 The results of fitting both models to the binary phase equilibria data and root-mean-square deviations sP, sT, sx and sy for binary mixtures
Mixture |
T/K |
Model |
a12/K |
a21/K |
sP/mmHg | sT/K | 103 sx |
103 sy |
methanol + benzene |
298.15 |
I |
22.70 |
1026.75 |
1.0 |
0.0 |
0.7 |
8.0 |
methanol + MTBE |
313.15 |
I |
–107.03 |
569.52 |
0.1 |
0.0 |
0.1 |
0.5 |
methanol + water |
298.14 |
I |
–160.39 |
158.59 |
0.1 |
0.0 |
0.6 |
4.0 |
MTBE + benzene |
313.15 |
I |
–142.60 |
199.40 |
1.9 |
0.1 |
0.8 |
5.0 |
water + benzene |
298.15 |
I |
765.18 |
1663.40 |
||||
water + MTBE |
298.15 |
I |
173.24 |
1196.10 |
I, modified UNIQUAC model.
II, extended UNIQUAC model.
Table 5 The results of fitting both models to the ternary LLE data at 25
ºCMixture |
Na |
Ternary parameters |
Deviationsd |
||
Ib |
IIc |
Ib |
IIc |
||
water + methanol + benzene |
14 |
t231 = –0.2628 t132 = –0.0785 t123 = –0.1311 |
t231=
0.0022 t132= –0.6935 t123 = 0.0539 |
1.61e |
3.05 |
water + methanol + MTBE |
6 |
t231 =
–0.0007 t132 = –0.0427 t123 = 0.0330 |
t231 =
0.1497 t132 = –0.0411 t123 = –0.4815 |
0.64 |
0.83 |
water + MTBE + benzene |
11 |
t231 =
0.0190 t132 = 0.1172 t123 = 0.1215 |
t231 =
–0.0140 t132 = 1.5224 t123 = –0.0882 |
0.33 |
0.34 |
a
N, no. of tie-lines. b I, modified UNIQUAC model. c II, extended UNIQUAC model.Table 6 The results of fitting both models to the quaternary LLE data at 25ºC
Mixture |
Na |
Quaternary parameters |
Deviationsd |
||
Ib |
IIc |
Ib |
IIc |
||
water + methanol + MTBE + benzene |
|
t2341 =0.1185 t1342 = –1.9860 t1243 = 2.7185 t1234 = 0.0350 |
t2341 = –0.0542 t1342 = –0.2141 t1243 =–11.7981 t1234 = 5.5108 |
1.89e |
3.44 |
a
N, no. of tie-lines. b I, modified UNIQUAC model. c II, extended UNIQUAC model.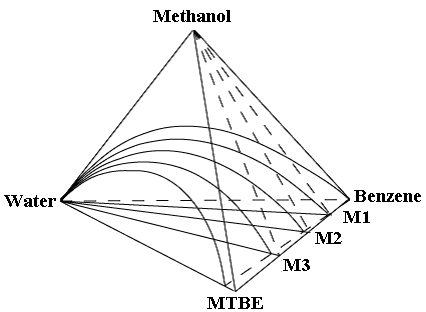
4. CONCLUSION
LLE data were measured for the ternary
mixtures of (water + MTBE + benzene) and quaternary mixtures of (water + methanol + MTBE +
benzene) at 25 ºC. The experimental ternary and quaternary LLE data were better
correlated by using both the extended and modified UNIQUAC models including binary,
ternary and quaternary parameters. The correlated results obtained by the models are
better than the predicted results, and show a good agreement with the experimental
quaternary LLE results.
REFERENCES
[1] Chen Y, Zhang S L. J Solution Chem,
2007, 36: 583-594.
[2] Gramajo de Doz M B, Cases A M, Sólimo
H N. Fluid Phase Equilibria, 2006, 249: 109-114.
[3] Chen Y, Dong Y H. J Chem Thermodyn, 2006, 38: 484-489.
[4] Chen Y, Dong Y H, Pan Z J. J Chem Thermodyn, 2005, 37: 1138-1143.
[5] Dong Y H, Chen Y. Chem J Internet, 2005, 7: 68-73.
[6] Nagata I. Fluid Phase Equilibria, 1990, 54: 191-206.
[7] Tamura K, Chen Y, Tada K, Yamada T, Nagata I. J Solution Chem, 2000, 29: 463-488.
[8] Hwang S C, Robert L, Robinson J. J Chem Eng Data, 1977, 22: 319-325.
[9] Toghiani R K, Toghiani H, Venkateswarlu G. Fluid Phase Equilibria, 1996, 122: 157-168.
[10] Kooner Z S, Phutela R C, Fenby D V. Aust J Chem, 1980, 33: 9-13.
[11] Oh J H, Han K J, Won D B, Park S J. Fluid Phase Equilibria, 2003, 209: 215-228.
[12] Sφrensen J M, Arlt W. Liquid–Liquid Equilibrium Data Collection, Vol. V, Part 1, DECHEMA,
Frankfurt am Main, 1979.
[13] Arce A, Blanco M, Riveiro R, Vidal I. Can J Chem Eng, 1996, 74: 419-422.
[14] Sφrensen J M, Arlt W. Liquid-Liquid Equilibrium
Data Collection, Vol. V, Part 2, DECHEMA, Frankfurt am Main, 1980.
[15] Arce A, Blanco A, Blanco M, Soto A, Vidal I. Can J Chem Eng, 1994, 72: 935-938.
[16] Prausnitz J M, Anderson T F, Grens E A, et al. Computer Calculation for
Multicomponent Vapor-Liquid and Liquid-Liquid Equilibria . Prentice-Hall: Englewood
Cliffs, NJ, 1980:19.
[17] Riddick J A, Bunger W B, Sakano T K. Organic Solvents; 4th ed.; Wiley-Interscience:
New York, 1986.
[18] Reddy S K V N, Dasika Prasad H L, Krishnaiah A. Fluid Phase Equilibria, 2005, 230:
105-108.
[19] Spencer C F, Danner R P. J Chem Eng Data, 1972, 17: 236-241.
[20] Hayden J G, O’Connell J P. J Ind Eng Chem
Process Des Dev, 1975, 14: 209-216.
[21] Nelder J A, Mead R. J Computer, 1965, 7: 308-313.
含二对部分互溶溶剂的四元体系-水、甲醇、甲基叔丁基醚、苯的液液相平衡