http://www.chemistrymag.org/cji/2008/105024ne.htm |
May 1,
2008 Vol.10 No.5 P.24 Copyright |
Zhang Xiaojuan, Sun Hongmei, Shen Qi, Zhang Yong
(Key Laboratory of Organic Synthesis of Jiangsu Province, Department of Chemistry and
Chemical Engineering, Suzhou University, Suzhou 215006, China)
Abstract Reaction of FeCl2(THF)1.5
with two equivalents of the lithium salt of 2,4-bis(4-methylphenylimido)pentane
(LLi) in toluene overnight at 80oC, afforded a bis-b-diketiminate Fe(II) complex of L2Fe (1) in high
yield. Attempt to synthesize the corresponding mono-ligand complex of iron by the same
reaction in the molar ratio of 1:1 [FeCl2(THF)1.5 : LLi] is
unsuccessful, the same complex 1 was obtained. Its structure was determined by
single-crystal X-ray diffraction. The iron atom is four-coordinated by four nitrogen atoms
of two diketiminate ligands to form a slightly distorted tetrahedral geometry.
Keywords Iron, b-diketiminate
ligand, Crystal structure, Synthesis
Supported by National Natural Science Foundation of China (No. 20772089) and the Key Laboratory of Organic Synthesis of Jiangsu Province.
1 INTRODUCTION
In recent years, the application of the chelating b-diketiminate ligands in coordination chemistry has attracted
significantly increasing attention, since their easily tuned in electronic and steric
effects by the diversity of nitrogen substituents[1]. In particular, bulky
aryl-substituted b-diketiminate
ligands have been widely used to stabilized three-coordinated iron complexes[2-7].
The bulky ortho substitutents on the aryl ring or the direct bulky substituent on the
nitrogen atom provide great steric hinderance very close to the unsaturated coordinated
metal. Furthermore, some of these complexes have shown some interesting catalytic
activities[2, 7-9]. On the contrary, the b-diketiminate ligands with small steric hinderance, i.e. the
aryl-substituted ones without the ortho substituents, have received little attention. In
order to elucidate the influence of ortho substituents on the molecular structure of the b-diketiminate-based iron complexes, we
used 2,4-bis(4-methylphenylimido)pentane as an ancillary ligand and synthesized the bis-b-diketiminate Fe(II) complex (1).
The results are reported as follows. By the way, two bis-b-diketiminate Fe(II) complex, i.e. containing ortho fluoro
substituent (2) on the aryl ring[3] or containing bulky substituent
(-SiMe3) (3) on the nitrogen atom[10], have recently been
reported as a byproduct[3] or a catalytic precursor for olefin polymerization[10],
respectively.
2 EXPERIMENTAL
2.1 General considerations
All manipulations were performed under pure argon with rigorous exclusion of air and
moisture using standard Schlenk techniques. Solvents were distilled from Na/benzophenone
ketyl under pure argon prior to use. 2,4-bis(4-methylphenylimido)pentane (HL)[11]
and FeCl2(THF)1.5[12] were prepared according
to the literatures. Elemental analysis was performed by direct combustion on a Carlo-Erba
EA-1110 instrument. The 1H NMR (400 MHz, C6D6) spectrum
was measured on a Unity Inova-400 spectrometer at 25oC.
2.2 Synthesis of L2Fe (1)
2.2.1 Procedure A
Schlenk flask was charged with 0.57 g (2.04 mmol) of HL and 30 mL toluene with a stir bar.
To this solution was added nBuLi (1.4 mL, 1.5 M, 2.04 mmol) in hexane solution
via syringe at 0oC. The light yellow solution stirred for 20 min at 0oC
and another 40 min at room temperature. Then, 0.23 g (1.02 mmol) of FeCl2(THF)1.5
was added to it. The mixture was stirred overnight at 80oC. The resulted
orange suspension was cooled at room temperature. After filtration, the solvent was
removed in vacuum and the residue was extracted with hexane. The filtrates were cooled at ﹣5oC. The complex 1 was obtained as orange
crystals. Yield: 0.47 g (75%). M.p. 194.3-195.2oC. Anal.Calc. (found) for C38H42FeN4
: C 74.09 (74.75), H 6.91 (6.93), N 9.15 (9.18) 1H NMR (400
MHz, C6D6, 25oC) : dppm,33.63, 20.21, 13.00,
3.61, 1.44,﹣51.24,﹣96.24.
2.2.2 Procedure B
The procedure is analogous to that described above, but the molar ratio of HL to FeCl2(THF)1.5
was 1:1. The same complex 1 was isolated with the yield of 35% (0.22 g).
2.3 X-ray crystallography
A suitable crystal with dimensions of 0.47 mm×0.37 mm×0.30 mm was sealed under argon in
a thin-walled glass capillary for X-ray structure analysis. Intensity data for 1 were
collected on a Rigaku Mercury CCD area detector using graphite monochromated MoKa
radiation (l = 0.71070
Å). Diffraction data were collected by w-2q scan technique. The total reflections of 53711 were collected, of
which independent reflections of 12112 could be observed and used in the structure
refinement. The structure was solved by direct method. The positions of all non-hydrogen
atoms were refined anisotropically with full-matrix least-squares on F2.
In the final difference map, the residuals are 0.296 and -0.277 e/Å3,
respectively.
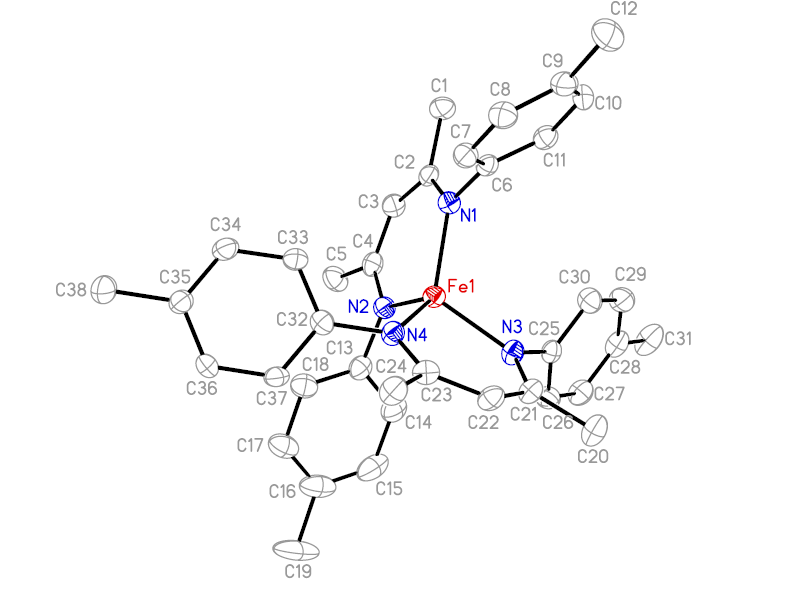
Fig.1 Molecular structure of 1.
3 RESULTS AND DISCUSSION
Reaction of FeCl2(THF)1.5 with two equivalents of the lithium salt
of 2,4-bis(4-methylphenylimido)pentane (LLi) in toluene overnight at 80oC,
after workup, produced the complex 1 as orange crystals in high yield. The
composition of this complex was established as L2Fe by elemental analysis and 1H
NMR spectrum. As shown in Scheme 1, attempt to synthesize the corresponding mono-ligand
complex of iron by the same reaction in the molar ratio of 1:1 [FeCl2(THF)1.5
: LLi] is unsuccessful. Still the bis-ligand complex 1 was isolated in
reasonable lower yields. This phenomenon is quite different from those observed in the
reaction of FeCl2(THF)1.5 with the lithium salts of bulky
aryl-substituted ligands, in which the three-coordinated Fe(II) complex of LFeCl or
four-coordinated one of LFe(m -Cl)2Li was isolated as the main product[13].
The present results indicate clearly that the ortho substituent on the aryl ring play an
great influence in the molecular structure of b-diketiminate-based iron complexes, and the absence of the ortho
substituent is of benefit to the formation of bis-β-diketiminate
Fe(II) complex. The complex 1 is possessed of good solubility and is well soluble
in hexane.
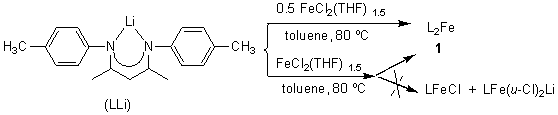
Scheme 1
The structure of the complex 1 is
shown in Fig.1. The crystallographic data and analysis parameters are shown in
Table 1. Selected bond lengths and angles are listed in Table 2.
Table 1 Crystal data and structure analysis parameters
Complex |
L2Fe |
Dc (g/cm3) |
1.218 |
Crystal size (mm3) |
0.47×0.37×0.30 |
F(000) |
2592 |
Empirical formula |
C38H42FeN4 |
Scan mode |
w-2q |
Temperature (K) |
223(2) |
q (°) |
3.01 -25.35 |
Wavelength Å) |
0.71070 |
Absorption coefficient (mm-1) |
0.484 |
Formula weight |
610.61 |
Reflections collected |
53711 |
Crystal system |
Orthorhombic |
Independent reflections |
12112 |
Space group |
Pca21 |
Goodness-of-fit |
1.082 |
a (Å) |
13.551(1) |
R [I>2s (I)] |
0.0546 |
b (Å) |
15.372(2) |
wR2 |
0.1003 |
c (Å) |
31.975(3) |
Maximum diff. peak (e/Å3) |
0.296 |
V(Å3) |
6660.5(12) |
Minimum diff. peak (e/Å3) |
-0.277 |
Z |
8 |
Table 2 Selected bond lengths (Å) and bond angles (°)
Bond lengths |
|||
Fe(1)-N(1) |
2.031(3) |
N(2)-C(4) |
1.336(5) |
Fe(1)-N(2) |
2.003(3) |
N(2)-C(13) |
1.435(5) |
Fe(1)-N(3) |
2.009(3) |
N(3)-C(21) |
1.326(5) |
Fe(1)-N(4) |
2.021(3) |
N(3)-C(25) |
1.435(5) |
N(1)-C(2) |
1.344(5) |
N(4)-C(23) |
1.341(5) |
N(1)-C(6) |
1.431(5) |
N(4)-C(32) |
1.426(5) |
Bond Angles |
|||
N(1)-Fe(1)-N(2) |
93.13(1) |
N(3)-Fe(1)-N(4) |
92.04(1) |
N(1)-Fe(1)-N(3) |
116.79(1) |
N(2)-Fe(1)-N(4) |
115.91(1) |
C(2)-N(1)-Fe(1) |
124.2(3) |
C(4)-N(2)-Fe(1) |
125.4(3) |
C(23)- N(4)- Fe(1) |
124.3(3) |
C(21)-N(3)- e(1) |
126.6(3) |
C(6)-N(1)-Fe(1) |
116.7(3) |
C(13)-N(2)-Fe(1) |
115.1(3) |
C(25)-N(3)-Fe(1) |
114.9(3) |
C(32)-N(4)-Fe(1) |
116.1(2) |
X-ray structure analysis reveals that the complex 1 is a bis-b-diketiminate Fe(II) complex, and the iron atom is bound to four nitrogen atoms of two b-diketiminate ligands to afford an FeN4 array that approximates D2d symmetry with an average Fe-N distance of 2.016(3) Å. The average Fe-N distance falls within the range previously reported values of 1.999(1)-2.113(3) Å[3, 10]. Furthermore, the Fe-N distance for each b-diketiminate ligand is slightly different from each other, such as the Fe-N(1) (2.031(3) Å) or Fe(1)-N(4) (2.021(3) Å) distance is longer than the Fe-N(2) (2.003(3) Å) or Fe-N(3) (2.009(3) Å), respectively. This difference is quite similar to those found in 2[3], and slightly smaller than those found in 3[10]. The dihedral angle between N(1)-Fe(1)-N(2) and N(3)-Fe(1)-N(4) is 86.67o, which is obviously larger than the value found in 3 (65.1o )[10]. The angles of N(1)-Fe(1)-N(2) and N(3)-Fe(1)-N(4) are relatively acute at 93.13o and 92.04o, while the angles of N(1)-Fe(1)-N(3) and N(2)-Fe(1)-N(4) are relatively obtuse at 116.79o and 115.91o, respectively. So it is a slightly distorted tetrahedral geometry around the iron atom. This type of coordination geometry presented in 1 is very similar to that found in 2[3], and obviously different from that found in 3[10]. These results are mainly due to bulky effect of β-diketiminate ligands, and indicate that the less steric bulky of the β-diketiminate ligand is of benefit to exert a more regular tetrahedral geometry around the iron atom. Besides, the dihedral angles between C(2)-C(3)-C(4) or N(1)-Fe(1)-N(2) and C(2)-C(4)-N(2)-N(1) are 1.26o and 0.92o, respectively. Meanwhile, the dihedral angles between C(21)-C(22)-C(23) or N(3)-Fe(1)-N(4) and N(3)-C(21)-C(23)-N(4) is 4.77o and 8.58o, respectively. These results mean that there is a little difference between the plane geometry of the two b-diketiminate ligands in one molecular structure of 1, and one of them is almost coplanar.
4. CONCLUTION
In summary, we have successfully synthesized a new soluble bis-b-diketiminate Fe(II) complex 1 in high yield, and
characterized its structural feature by X-ray crystallography. In comparison with the
reported results[3, 10, 13], the present work provides a direct evidence to
elucidate that the ortho substituent on the aryl ring of bis-b-diketiminate ligands plays a great influence on the molecular
structure of the b-diketiminate-based
iron complexes.
REFERENCES
[1] Bourget-Merle L, Lappert M F, Severn J R. Chem. Rev., 2002, 102: 3031.
[2] Gibson V C, Marshall E L, Navarro-Llobet D et al.Dalton Trans., 2002, 4321.
[3] Panda A, Stender M, Wright R J et al. Inorg. Chem., 2002, 41: 3909.
[4] Eckert N A, Smith J M, Lachicotte R J et al. Inorg. Chem., 2004, 43: 3306.
[5] Vela J, Vaddadi S, Cundari T R et al. Organometallics, 2004, 23: 5226.
[6] Gregory E A, Lachicotte R J, Holland P L. Organometallics, 2005, 24: 1803.
[7] Vela J, Smith J M, Yu Y et al. J. Am. Chem. Soc., 2005, 127: 7857.
[8] Smith J M, Sadique A R, Cundari T R et al. J. Am. Chem. Soc., 2006, 128: 756.
[9] Yu Y, Brennessel W W, Holland P L. Organometallics, 2007, 26:
3217.
[10] Zhou M S, Huang S P, Weng L H et al. J. Organomet. Chem., 2003, 665: 237.
[11] Feldman J, McLain J S, Parthasarathy A et al. Organometallics, 1997, 16: 1514.
[12] Kern R J. J. Inorg. Nucl. Chem., 1962, 24: 1105.
[13] Smith J M, Lachicotte R J, Holland P L. Chem. Commun., 2001, 1542.
张晓娟,孙宏枚,沈琪,张勇
(江苏省有机合成重点实验室,苏州大学化学化工学院,苏州215006,中国)
摘要 通过b-二酮亚胺的锂盐和FeCl2(THF)1.5的2:1(摩尔比)反应,我们以较高的产率得到了双b-二酮亚胺铁配合物1。但是,通过两者1:1(摩尔比)的反应,我们没能分离到预期的单配型的b-二酮亚胺铁的氯化物。配合物1通过了元素分析、氢谱和X-衍射等表征。结构测定表明,中心金属原子Fe与b-二酮亚胺上的四个氮原子配位,形成了一个略扭曲的四面体。本文的研究结果表明b-二酮亚胺芳基上邻位-取代基对相应铁配合物的组成和结构有很大的影响。
关键词 二价铁、b-二酮亚胺、晶体结构、合成