http://www.chemistrymag.org/cji/2008/10a052ne.htm |
Oct.
4, 2008 Vol.10 No.10 P.52 Copyright |
Du
Yanjun, Zheng Yanju, Du Baoan, Zhang Jingfeng
(College of Chemistry and Environmental Science, Hebei University, Baoding 071002, China)
Abstract Nanosized ZnFe2O4
powders were prepared by co-precipitation method using ZnSO4·7H2O
and FeCl3·6H2O as raw materials. The composition of crystal
phase and the sizes of particles were characterized by X-ray diffraction (XRD) and
transmission electron microscopy (TEM). The effect of pH value, concentration of reagents,
and ultrasonic on preparation were studied. Finally, and the optimum conditions were
obtained by comparison experiment.
Keywords co-precipitation method; preparation; nanosized ZnFe2O4
1. INTRODUCTION
Due to its paramagnetic property, ZnFe2O4 possessing normal
spinel structure is widely used for industrial materials including magnetic materials, gas
sensor, absorbent material for hot-gas desulphurization, and semiconductor photo catalyst.[1-4]
Traditionally, nanosized ZnFe2O4 is prepared by wet-milling process[1],
Hydrothermal synthesis[2], solvent thermal process[3], shock wave
treatment[5], sol-gel method[6], solid state reaction[7],
combustion synthesis[8]. However, all these methods are very complicated for
obtaining nanosized ZnFe2O4.
A new technique for preparation of nanosized ZnFe2O4 powders
by co-precipitation method using ZnSO4·7H2O and FeCl3·6H2O
as raw materials is reported in this study. The most important advantage of this technique
is its feasibility for large-scale production as well as its simplicity and low cost.
2. EXPERIMENTAL
A series of FeCl3 solutions were obtained by dilution: 0.2, 0.4, 0.6, 0.8,
1.0 mol× L-1. And the ZnSO4 solutions were also achieved: 0.1, 0.2,
0.3, 0.4, 0.5 mol× L-1. The FeCl3 solution and the ZnSO4
solution were mixed in a beaker with a molar ratio of 1(Zn):2(Fe). 1 mol× L-1
NaOH was used for adjusting the pH value of the mixture ranged from 8 to 13. Then, the
mixture was stired for 30 min, decompression filtered, washed by distillated water 3
times, and then washed by ethanol 2 times. The filter cake was exposed to the air for 24
hrs of dehydration naturally. Then the precursor was obtained. The precursor was calcined
at 973 K for 6 h. Finally, the products were obtained. The phase compositions of the
products were examined by XRD. The sizes and shapes of the products were testified by TEM.
3. RESULTS AND DISSCUSSION
3.1 Optimization of the preparation
The pH of the reaction system plays an important role in the research. When the pH was
less than 8, Zn(OH)2 was not precipitated completely, then the Zn:Fe was less
1:2; when the pH was larger than 9, it was difficult to wash and leach the precipitate,
the products was hard for aggradation. Finally, the results showed that pH value ranged
from 8 to 9 is the best choice.
The effect of concentrations of solution for preparation was also very
important. The capacity for preparation become larger when the concentration becomes
lower, and then the production cost become also much higher. The higher of the
concentrations, the impurities in product were much more. Finally, the optimum
concentration of the raw materials were FeCl3 0.6 mol× L-1 and ZnSO4
0.3 mol× L-1.
3.2 XRD analysis of the ZnFe2O4 nanoparticles
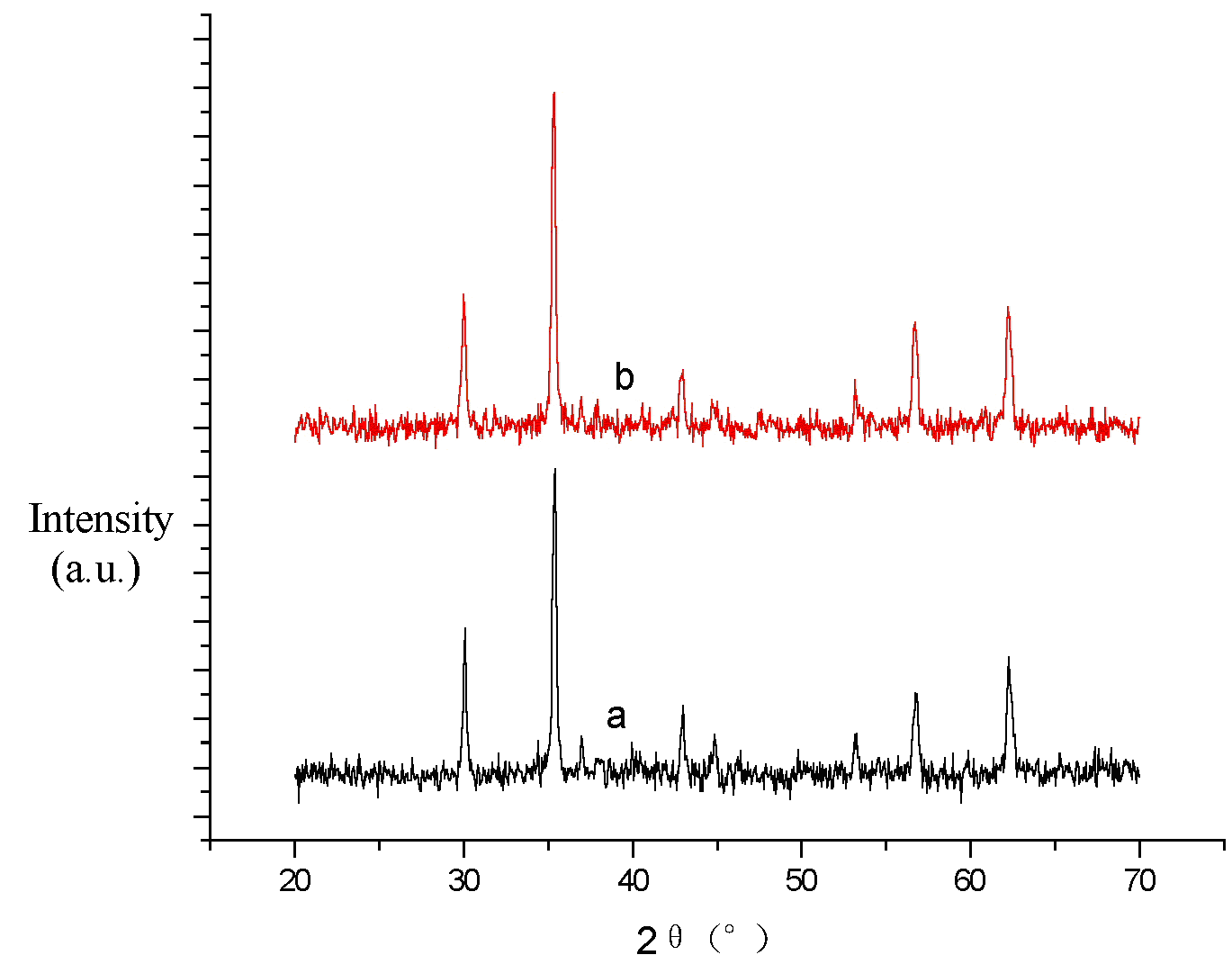
Fig. 1 XRD patterns of the products
(a) dispersed in ethanol and treated by ultrasonic for 30min and
(b) not treated by ultrasonic. FeCl3: 0.6 mol× L-1; ZnSO4:
0.3 mol× L-1; pH = 9.
The average sizes of particles were calculated by the Scherrer formula that was D = k·l/b·cosq (D/nm: the primary diameter of the product; k: 1.075; lCu/nm: 0.154178; b/arc: integral peak width; q /degree: angle of diffraction). Many X-ray diffraction peaks of low angular degree (generally 2q ≤50°) were selected for calculation, and the average value was obtained. According to the calculation, the primary diameter of the sample a was 20.6 nm, and the primary diameter of the sample b was 21.2 nm.
3.3 TEM analysis of nanosized ZnFe2O4 nanoparticles
The TEM photo of ZnFe2O4 particles was illustrated in Fig. 2. According to the photo, the shapes of the product were ball-like, the primary diameters of the particles were 20-30 nm, the deposition still existed, and the sizes of the deposition were about 50 nm. The result observed by TEM was consistent with the calculated value by the Scherrer formula.
Fig. 2 TEM photograph of the
sample
(×50000 times; FeCl3: 0.6 mol× L-1; ZnSO4: 0.3 mol× L-1;
pH = 9.)
3.4 Mechanism of the preparation of nanosized
ZnFe2O4 by co-precipitation method
The mechanism of prepared nanosized ZnFe2O4 powders can
be divided into the follow two steps. Firstly, the highly dispersed and easily decomposed
precursor was prepared; secondly, the precursor was thermal decomposed.
Co-precipitation process
includes single-phase co-precipitation process and multiple-phase
co-precipitation process. When the precipitation is simplex compound or single-phase solid solution, the process is called single-phase co-precipitation process, the ions precipitate at the same
time. When the precipitation is mixture, the process is called multiple-phase
co-precipitation process, the order of precipitating of ions had no effect on the form of
the precipitation. In the study, the color of the precursor prepared by hydrolyzed FeCl3·6H2O and the color of the
precursor prepared by FeCl3·6H2O without hydrolyzation was
different. In other words, the precipitation of ferric ion in advance affects the form of
the precipitation. So it could be concluded that the process of the technology is single-phase co-precipitation process.
4. CONCLUSION
Nanosized ZnFe2O4 powders were prepared by co-precipitation
method using ZnSO4·7H2O and FeCl3·6H2O
as raw materials. According to XRD and TEM, the products were nanosized ball-like ZnFe2O4.
The best conditions were obtained: FeCl3: 0.6 mol× L-1;
ZnSO4: 0.3 mol× L-1; pH = 9. The technique of
ultrasonic had no obvious effect on the products. The technology of prepared nanosized
ZnFe2O4 powders by co-precipitation method
REFERENCES
[1] Sadan Ozcan, Burak Kaynar, Musa Mutlu Can, Tezer Firat. Materials Science and
Engineering B, 2005, 121: 278–281.
[2] Shu-HongYu, Takahiro Fujino, Masahiro Yoshimura. Journal of Magnetism and Magnetic
Materials, 2003, 256: 420–424.
[3] ZHANG Yuanguang, CHEN Youcun, ZHOU Gentao. Chinese of Applied Chemistry, 2003, 20(4):
332-335.
[4] TIAN Qinghua, HUANG Kai, GUO Xueyi. Materials Science and Engineering of Powder
Metallurgy, 2005, 10(4): 200-204.
[5] XU Kang, LIU Jianjun, XU Tao. Journal of Inorganic materials, 1997, 12(5): 759-762.
[6] SHI Xiao-bo, LI Chun-gen, WANG De-xian. Chemical World, 2002, 43(9): 451-453.
[7] NIU Xin-shu, LIU Yan-li, XU Jia-qiang,
JIANG Kai. Journal of Functional Materials, 2002, 33(4): 413-414,
417.
[8] JIANG Jiuxing, LI Yao, HE Xiaodong, QU Wei.
Journal of the Chinese Ceramic Society, 2003, 31(3): 235-240.
杜艳君 ,郑艳菊,杜宝安,张景峰
(河北大学化学与科学学院,保定,071002)
摘要 以ZnSO4·7H2O和FeCl3·6H2O为原料,利用共沉淀法制备纳米铁酸锌粉体。应用XRD和TEM技术研究了铁酸锌晶体的组成、粒径大小及粒子形貌。对影响合成工艺的因素,包括反应的pH值、反应物浓度、超声手段等进行了系统的考察研究,确立了制备纳米铁酸锌的最佳条件。
关键词 共沉淀法;制备;纳米ZnFe2O4