http://www.chemistrymag.org/cji/2009/112008pe.htm |
Feb.1,
2009 Vol.11 No.2 P.8 Copyright |
Shen
Shigang, Hao Zenghua, Shi Hongmei, Song Changying
(College of Chemistry and Environmental Science, Hebei Uinversity, Baoding 071002, Hebei
Province, China)
Abstract The kinetics of substitution of tetrachloroaurate (III) by L-Glutamine has been spectrophotometrically studied in acid medium. Under pseudo-first-order conditions ([R]0>>[Au(III)]0) is first order in [Au(III)]0 and a fractional order in [R]0. Both H+ and Cl- ions retard the reaction. The kobs is independent of ionic strength μ. A substitution reaction mechanism is proposed, the reaction of Au (
III) with R includes two parts, the first phase is a preequilibration, which takes place between the cationic and the zwitterion species of R with AuCl4-, and forms an intermediate complex AuCl3RH. The second phase this unstable intermediate AuCl3RH slowly decomposes to give a half-cyclic Au (III) complex that appears to be unreactive, it be conformed as the rate determining reaction. The rate limiting constant k, and its thermodynamic parameters are also reported (which: the L-Glutamine= R, the cationic of L-Glutamine = RH2+ and the zwitterion of L-Glutamine = RH).Keywords Tetrachloroaurate (III); L-Glutamine; Substitution; Kinetics and mechanism
1. INTRODUCTION
The chemistry of gold complexes is important from a biological and medicinal point of
view Au (III) is isoelectronic and isostructural to platinum (II) [1, 2], therefore, its complexes have long been evaluated as
potential anticancer agents due to the structural similarity to platinum-based drugs [3].
The hydrolysis of AuCl4- has been studied by several investigators
and the existence of different Au (III) species has been suggested [4,
5]. Au (III) is a low spin d8 centre which forms
square-planar complexes. With a lowly acid medium, Au (III) as an
oxidant is of interest as it may behave either as a one- or two-electron transfer oxidant.
But the high acid medium of the Au (III) complexes limits the number
of nucleophiles can be investigated without complications from substitution reaction
[4-6]. However, chloride complexes of Au (III) are
thermodynamically stable in aqueous solutions and also labile [7-12]. It is
therefore ideal candidates for a fundamental study involving kinetic [13-17].
The kinetics of the oxidation of Glycolaldehyde [8], Sugars [18],
O-phenylenediamine [19], Oxalic acid [20], Hydrazoic
[21] by Au (III) have already been reported in recent times.
The present investigation on the substitution of Au (III) by
L-Glutamine in perchloric acid medium was carried out in order to throw light upon the
reactivity of L-Glutamine towards Au (III), Since AuCl4- gets
partially hydrolysed [3-5] and it is difficult to keep [H+] constant
when change the substrate concentrations at lower acidities, but the reactions are too
slow to be studied in a highly acidic medium ([H+]>0.1mol dm-3), the kinetics of the reaction have been
monitored in perchloric acid medium where the reaction occurs at ameasurable rate.
Furthermore, the result is very interesting, because it is very different from with the
mechanism as reported for several analogous systems [15-17]. The meaning is
that the reaction of Au (III) with L-Glutamine is a substitution reaction which is
dominant from the tracked datas, furthermore, no kinetic data are available in the
literature on the substitution of amino acids by Au (III).
2. EXPERMENTAL SECTION
2.1 Chemicals and solutions
L-Glutamine was obtained from guo yao ji tuan Chemical Reagent Company. NaAuCl4
was purchased from Alfa Aesar. NaCl, NaClO4 and HClO4 were obtained
either from Beijing Chemical Reagent Company (Beijing, China) or from Tianjin Chemical
Reagent (Tianjin, China). All the above reagents were of either analytical grade or
reagent grade and used as received. All solutions were prepared with doubly distilled
water.
2.2. Spectral and kinetic measurements
Electronic spectral recordings and kinetic measurements were performed on a UV–visible spectrophotometer (TU-1901, Beijing Puxi, Inc.,Beijing,
People's Republic of China), and quartz cells with a 1.00 cm optical pathlength were used.
The spectrophotometer was equipped with a cell compartment which was thermostated by a
thermostat (BG-chiller E10, Baijing Biotect Inc., Beijing Beijing, China). Temperature of
solutions in cells can be controlled to ±0.2℃ when
cells are put in the compartment. Two reaction solutions, one containing known
concentrations of NaAuCl4、HClO4、NaCl, and the other containing desired concentrations of R and
NaClO4, were thermostated for at least 20 min before mixing each other. The
function of NaClO4 was to adjust the ionic strength (m) in the reaction solutions. The HClO4 was used to
adjust the pH. Pseudo first-order reaction conditions were fulfilled by making [R]0
≥ 100[Au(III)]0. Kinetic traces were
followed at 313 nm by the same spectrophotometer mentioned above. Time-resolved spectra
for a typical reaction between Au (III) and R are displayed in (Fig. 1); no apparent shift
of the absorption peak around 313 nm indicated that concentrations of any reaction
intermediates in the reaction course could be very low.
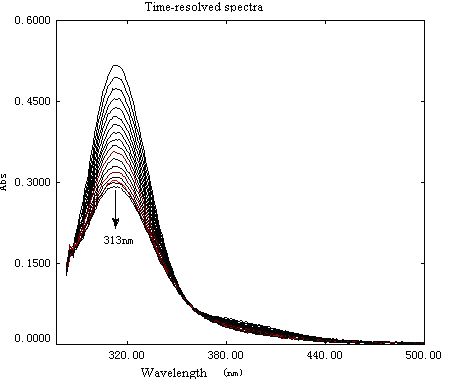
Fig. 1 Time-resolved spectra for reaction between Au (III) and R at 298.2 K. [Au (III)]0=1.00×10-4mol·L-1, [R]0 =5.00×10-2mol·L-1, [H+]=2.00×10-3mol·L-1, [Cl-]=2.60×10-2mol·L-1 and
m=0.30 mol·L-1.2.3. Product analysis
The reaction mixture (concentration of each reactant being twenty times that under kinetic
condition) was allowed to stand for two hours, and then distilled, the distillate being
collected in a closed container. Then the distillate was treated with a small amount of
gallic acid and a few drops of conc H2SO4 and heated on a water
bath, these is mostly nothing appeared [22], indicating no presence of RCHO.
In another experiment, The reaction mixture containing 25% (V/V)
acrylonitrile was allowed to stand for two hours under the protection of nitrogen gas,
these is mostly nothing appeared, No polymerization of acrylonitrile was observed, showed
that there is no free radical intermediate appeared.
3. RESULT AND DISCUSSION
3.1 Kinetic
3.1.1 Reaction orders
The rates were measured under pseudo first-order conditions ([R]0 >> 100 [Au (III) ]0) at constant ionic strength (μ=0.3mol L-1) by monitoring the changes at 313 nm. Plots of Ln[(A0- A∞)/(At-A∞)]versus reaction time were linear and pass through the origin under these conditions (Fig. 2), suggesting that the reaction is first order in [Au(III)]0, where At and A∞ refer to absorbance at time t and infinity, respectively (Eqn. 1).
Pseudo-first-order rate constants kobs were calculated from slopes of these linear. Values of kobs, representing an average of three repeated runs, are generally reproducible to ±5% within standard deviations.
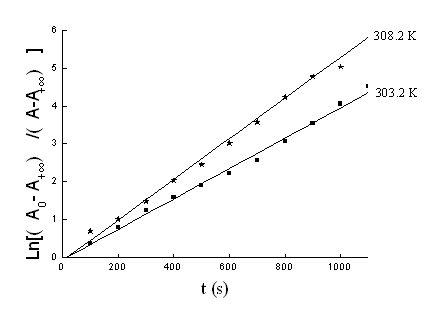
Fig. 2 Ln [(A0- A∞)/ (At-A∞)]vs reaction time t at different temperatures. [Au (III)]0=1.00×10-4mol·L-1, [R]0 =5.00×10-2mol·L-1, [H+]=2.00×10-3mol·L-1, [Cl-]=2.60×10-2mol·L-1 and μ=0.30 mol·L-1.
3.1.2 Rate dependence on [R]0
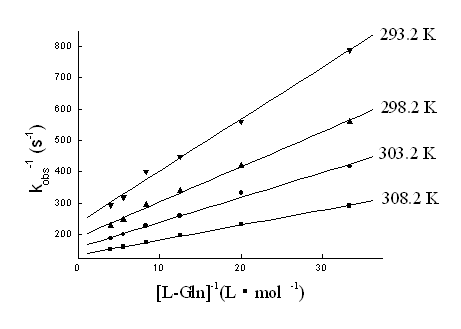
Fig. 3 The plots of kobs-1 vs [R]0-1 at different temperatures. [Au (III)]0=1.00×10-4mol·L-1, [H+]=2.00×10-3mol·L-1, [Cl-]=2.60×10-2mol·L-1 and μ=0.30 mol·L-1.
3.1.3 Rate dependence on [H+]
The rate of the reaction was studied at different pH values (1.90-3.00) using perchloric
acid .The value of kobs was found to increase with an increase in pH. The plot
of kobs-1 vs [H+] gave a straight line with a positive
slope and positive intercept. (Fig. 4).
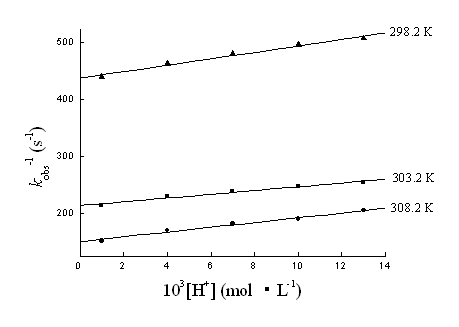
Fig. 4 The plots of 1/kobs vs [H+] at different temperatures. [Au (III)]0=1.00×10-4mol·L-1, [R]0 =5.00×10-2 mol·L-1, [Cl-]=2.60×10-2 mol·L-1 and μ=0.30 mol·L-1.
3.1.4 Rate dependence on [Cl-]
The reaction was studied at different temperatures at different [Cl-] varied by
the addition of NaCl. The value of kobs was found to decrease with an increase
of [Cl-]. The plot of kobs-1 vs [Cl-] is
linear with a positive slope and positive intercept (Fig. 5).
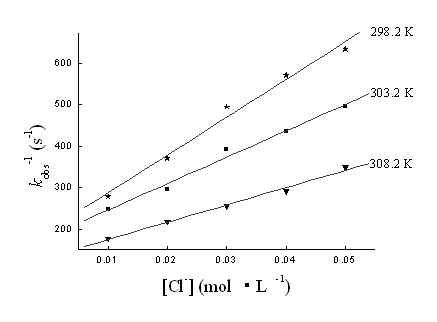
Fig. 5 The plots of kobs-1 vs
[Cl-] at different temperatures. [Au (III)]0=1.00×10-4mol·L-1,
[R]0=5.00×10-2mol·L-1, [H+]=2.00×10-3mol·L-1
and μ=0.30mol·L-1.
3.1.4 Rate dependence on ionic
strength m
The effect of ionic strength was studied by varying the NaClO4 concentration in
the reaction medium. The ionic strength μ of the
reaction medium was varied from 0.10 to 0.53 M. It was found that varied ionic strength
did not show any effect on the rate of reaction (Table 1).
Table 1. The effect of the ionic strength on the rate of the reaction at 298.2 K
ClO4-/(mol·L-1) |
0.10 |
0.20 |
0.30 |
0.40 |
0.53 |
m/ (mol·L-1) |
0.23 |
0.33 |
0.43 |
0.53 |
0.63 |
103kobs (s) |
2.09 |
2.09 |
2.07 |
2.19 |
2.16 |
[Au (III)]0=1.00×10-4mol·L-1,[R]0=5.00×10-2mol·L-1,[H+]=2.00×10-3mol·L-1and [Cl-]=2.60×10-2 mol·L-1.
3.2 Discussion
3.2.1 Protolytic equilibria
The ionization of L-glutamine occurs as:
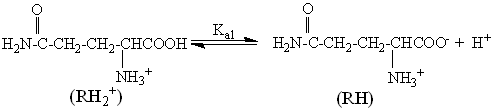
Ka1=6.76×10-3 (2)
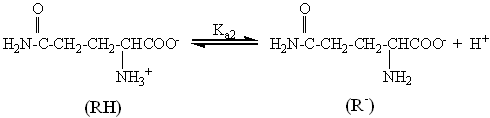
Ka2=7.41×10-10 (3)
It may be noted that most
of the experiments were carried out at a pH of 2.70, where [RH2+]:
[RH] is 1:3.38, the equilibrium (2). Sadler et al. studied [3] the authors
proposed a reaction mechanism with the involvement of the cationic and the zwitterion
species of R. It is evident that the concentration of R- is too small to be
considered as a reaction species. It may be noted that at the above mentioned pH, the
cationic and the zwitterion species will be present as a mainly species evidently from the
pKa value of R [23]. Therefore, two species are most likely to be present as
the predominant and effective substituting species under the conditions of the experiment.
The ionization of Au (III) occurs at 298 K as below:
![]() Khy=9.5×10-6 (4)
Khy=9.5×10-6 (4)
![]() Ka=0.25 (5)
Ka=0.25 (5)
Tetrachloroaurate (III) AuCl4 - ion exists in
equilibrium with other Au (III) species as shown in the equilibrium (4, 5). The
equilibrium (4) is helpful in explaining the rate retardation by Cl- ions on
the assumption that AuCl4- ion is much more than AuCl3(H2O).
Similarly, the equilibrium (5) can explain the retardation of the rate by H+
ions by considering AuCl3(OH-) is larger than AuCl3(H2O)[8].
Thus under the experimental conditions and utilizing the literature values of Khy
and Ka at 298 K, the ratio of concentration of the different Au (III) species
are found to be [AuCl4-]: [AuCl3(H2O)] is
2736:1, that the equilibrium (5) can be neglected. It may be proposed that AuCl4–
is the predominant and effective Au (III) species [21].
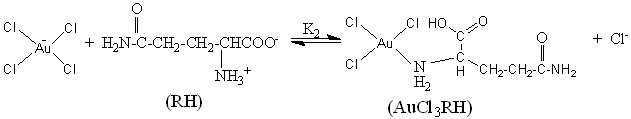
3.2.2 Mechanism prevails
Based on the kinetic results and analysis, a straightforward reaction mechanism is
proposed as below:
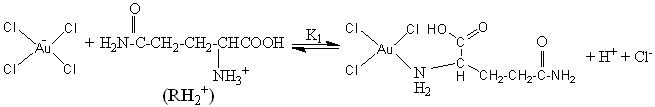 (6)
(6)
 (7)
(7)
 (8)
(8)
From the proposed reaction
mechanism, it is known that the reaction of Au (III) with R includes two phases, the first
phase is a preequilibration, it is quite plausible that the reaction takes place between
the cationic and the zwitterion species of the R with AuCl4- in the
equilibrium (6 and 7), and each molecule of RH2+ and RH consume a
molecule of AuCl4– respectively,
and form an intermediate complex AuCl3RH. It is different from the reported
reaction mechanism involvement of the two oxidizing species in the Au (III)-induced
oxidation of amino acids by Pratik K. Sen [22] and Vimal Soni [20].
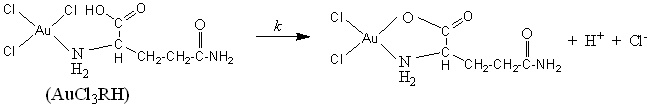
The second phase, this unstable intermediate AuCl3RH slowly decomposes to give
a half-cyclic Au (III) complex in the reaction (8). Once formed, the chelate half-cyclic
Au (III) appears to be unreactive. Several authors have also reported [3, 24] the
formation of an unreactive half-cyclic Au (III) intermediate complex in the substitution
of organic substrates by Au (III).
Consideration of the equilibriums (2) and (3) suggests that [RH2+]
and [R]0 are given by the (Eqn. 9 and 10):
![]() (9)
(9)
![]() (10)
(10)
According to the equilibriums (6) and (7), [AuCl4–] concerned two parts:
![]()
![]()
![]() (11)
(11)
Add two parts of [AuCl4–] in
the (Eqn. 11) get that the concentration total of AuCl4–:
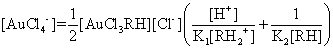 (12)
(12)
From the (Eqn. 13), get that the total of [Au (III)]0 involves [AuCl4–] and [AuCl3RH],
![]() (13)
(13)
Take the (Eqn. 9, 10 and 13) into (12) and Simplificated, the substitution of the values
of [AuCl3RH] from the (Eqn. 6 and 7) can show as fllowed the (Eqn.14):
![]() (14)
(14)
Under the pseudo first-order condition, the rate law derived from the disappearance of the
total concentration of Au (III) in terms of reactions (6-8) is given by (Eqn. 1). It can
be expressed as:
![]() (15)
(15)
![]() (16)
(16)
Unite (Eqn. 1). we can get the expression of kobs as the (Eqn. 17)
![]() (17)
(17)
The equation (17), which is the derived rate law, can be expressed by the equation (18-20)
![]() (18)
(18)
![]() (19)
(19)
![]() (20)
(20)
The equation (18-20) is consistent with the linear plots with
intercepts shown in Fig. 3 (kobs-1 vs [R]o-1),
Fig. 4 (kobs-1 vs [H+]) and Fig. 5 (kobs-1
vs [Cl-]).The agreement between the equation (18-20) and the results justifies
the proposed mechanism.The values of k at different temperatures were calculated from the
intercepts of kobs-1 vs [R]o-1, kobs-1
vs [H+] and kobs-1vs [Cl-] in different ways,
and these have been found to tally basely with each other.
3.2.3 Tthermodynamic parameters
From the intercepts of the linear plot of kobs-1 vs [R]0-1,
the values of k at different temperature were evaluated. The plot of log (k/T) vs (1/T) [18]
was found to be linear (r=0.997) and from the slope of this plot, the overall enthalpy of
activation (DH≠) followed by the overall entropy of activations (DS≠) were calculated using the relation (Eqn. 21) [8, 20].
Rate constants and activation parameters are listed in Table 3.
![]() (21)
(21)
Table 3. The values of k at the different temperatures.and the respective values of
the thermodynamic parameters.
T/K |
293.2 |
298.2 |
303.2 |
308.2 |
Activation parameters(298.2K ) |
103k/(L·mol-1 s-1) |
4.20 |
5.21 |
6.25 |
7.39 |
Ea = 30.43kJ·mol-1 |
3.3 Conclusion
In conclusion, a nucleophilic
substitution reaction occurred between amino acids and Au (III) complexes under the reaction conditions. The
cationic and the zwitterionic species of R react with Au (III) to give a half-cyclic compound in the pH range 1.8-3.0.
AuCl4- is more labile than the corresponding aqua forms, AuCl3H2O,
as expected. The reaction is a first order in [Au (III)]0, a fractional order in [R]0 and
negative fractional order with respect to H+ and Cl-. The kobs is
independent of ionic strength m. Therefore, it is an unambiguous indication that a
different mechanism involving some selected L-glutamine species are presently involved.
3.4 Acknowledgement
Financial support of this work in part by a grant from the Natural Science Foudation
of Hebei Province (B2006000962) is gratefully acknowledged.
REFERENCES
[1] Pengfei Shi, Qin Jiang, Yongmei Zhao, et al. J. Biol. Inorg. Chem, 2006, 11: 745.
[2] Pengfei Shi, Qin Jiang, Jun Lin, et al. J. Inorg. Biochem, 2006, 100: 939.
[3] Zou J, Guo Z, Parkinson JA, Chen Y, Sadler PJ, et al. Chem. Commun, 1999, 1359.
[4] Florence J. Monlien, Lothar Helm, et al. Inorg. Chim. Acta, 2002, 331: 257.
[5] Peter Schwerdtfeger, et al. J. Am. Chem. Soc, 1989, 11: 7262.
[6] Lise Drougge, Lars I. Elding, et al. Inorg. Chem, 1987, 26: 1073.
[7] Lars I. Elding, L. H. Skibsted, et al. Inorg. Chem, 1986, 25: 4084.
[8] John Wiley Sons, et al. Int. J. Chem. Kinet, 1998, 30: 613.
[9] Kalyan K. Sen Gupta, Biswajit Pal, Pratik K. Sen, et al. Int. J. Chem. Kinet, 1999,
31: 873.
[10] Annapurna J. Canumalla , Norah Al-Zamil, et al. J. Inorg. Biochem, 2001, 85: 67.
[11] Pal B, Sen PK, Sen Gupta KK, et al. J. Phys. Org. Chem, 2001, 14: 284.
[12] C. Frank Shaw III, M. P. Cancro, et al. Inorg. Chem, 1980, 19: 3198.
[13] Sofi Elmroth, L. H. Skibsted, Inorg. Chem, 1989, 28: 2703.
[14] Johan Berglund, Lars I. Elding, et al. Inorg. Chem, 1995, 34: 513.
[15] Sen Gupta. K. K., Begum. B. A, et al. Carbohydr. Res, 1999, 315: 70.
[16] Vimal Soni, R.S. Sindal and Raj N. Mehrotra, et al. Polyhedron, 2005, 24: 1167.
[17] Kensuke Fujiwara, Attinti Ramesh, et al. J. Hazardous. Materials, 2007, 146: 39.
[18] K.K. Sen Gupta, et al. Carbohydrate. Research, 2001, 330: 115.
[19] Ozlen Altuna,
Halide Akbas, et al. Molecular and Biomolecular. Spectroscopy, 2007, 66: 499.
[20] Vimal Soni, R.S. Sindal, Raj N. Mehrotra, et al. Inorg. Chim. Acta, 2007, 360: 3141.
[21] Vimal Soni, Raj N. Mehrotra, et al. Transition. Met Chem, 2008, 33: 367.
[22] Pratik K. Sen, Nasimul Gani, Jayanta K. Midya, et al. Transition. Met Chem, 2008, 33:
229.
[23] Owen BB, et al. J. Am. Chem. Soc, 1934, 56: 24.
[24] Sen Gupta KK, Pal B, Sen PK, et al. Int. J. Chem. Kinet, 1999, 31: 873.
申世刚,郝增华,石红梅,宋常英
(河北大学化学与环境科学学院,河北,保定,071002)
摘要 本文介绍了酸性条件下,不同温度下的Au(III)与L-谷氨酰胺的取代反应动力学及其机理,在准一级条件下([R]0 >> [AuCl4 -]0),反应对于Au(III)是一级的;对R是正分数级;H+ 和Cl- 都阻碍了反应,对反应是负数级;离子强度μ对反应无明显影响,忽略不计。从实验数据可以看出:该反应是一个取代反应,反应有两个前期平衡,主要是由AuCl4-与R的两种离子形式完成的,生成了中间产物AuCl3RH,由于AuCl3RH不稳定,进一步生成半环状的Au(III)化合物,这种半环状化合物很稳定,这一步反应很慢,作为反应的速控步。本文求出了速率常数k和各种活化参数(Ea、DH≠和DS≠)。(其中:L-谷氨酰胺表示为R, 它的阳离子形式为RH2+, 两性离子形式为RH).
关键词 Au(III);L-谷氨酰胺;取代反应;动力学及机理