http://www.chemistrymag.org/cji/2009/115024ne.htm |
May.1,
2009 Vol.11 No.5 P.23 Copyright |
Penta-O-benzoyl-D-glucose: an efficient glycosyl donor for the synthesis of a
-arbutin and a-aromatic-O-glucosidesFang Tao, Feng Weiwei, Zhang Mingdao, Fang
Zhijie
(School of Chemical Engineering, Nanjing University of Science & Technology, Nanjing
210094, China)
Abstract
a-arbutin, an inhibitor of tyrosinase is characterized as a- aromatic glucoside. The paper reports a facile glycosylation between penta-O-benzoyl-D-glucoside 3 and a series of aromatic aglycons 4a-4f catalyzed by BF3·Et2O forming a-arbutin intermediate 5a and other aromatic glucosides 5b-5f with good yields 91% and 75%-96%. Deprotection of 5a gives a-arbutin in 66% yield over 4 steps from glucose. Both the yield and stereoselectivity (a/b=6.2) exceed the present method. The hitherto unknown compounds 5a-5f are determined by 1H-NMR and EI-MS.Keywords a-Arbutin; Penta-O-benzoyl-D-glucose; 1, 2-cis-O- glycosylation; Stereoselective synthesis b-arbutin 1 is a nature product that extracted from blueberry leaves. Recently its synthetic anomer a-arbutin 2 has attracted more interests for its low histotoxicity [1] (IC50=2.1, b-arbutin IC50≥30). Therefore a-arbutin has prospective future in medical and cosmetic application [2].
Fig. 1 Structures of
The stereoselective formation of 1,2-cis-glycosidic linkage and the poor nucleophilicity of phenols under glycosylation condition are two aspects that challenge the synthesis of α-arbutin [3]. Its selective synthesis requires extra efforts due to neighboring participation, solvent effect, steric hindrance, and catalyst as well as temperature and reaction time [4]. Present method [5], starting from methyl glucoside only affords α-arbutin in 32% yield (
a/b=5). Such a low overall yield is mainly due to the further modification of penta- benzoyl glucoside to provide C-2 none participated and anomeric center activated trichloroacetimidate with yield 40%~50% by two steps from methyl glucose. Therefore the study of efficient 1, 2-cis-O-glycosylation not only contributes to the industrial application but also the synthesis methodology.Traditionally, per-benzoylated glucoside is so "disarmed" that it is rarely used as glycosyl donor. To our best knowledge, there are only two related documents [6, 7] reporting its application in aromatic glycosylation. In contrast, another kind of glycosyl ester: glycosyl acetate is widely used, but 1, 2-trans glycoside is preferred. Attracted by the easy accessibility of penta-O-benzoyl-D-glucose compared with the multi-steps and low yield of preparing C-2 non-participating group protected trichloroacetimidate glycosyl donor, we reconsider the application of penta-O-benzoyl-D-glucoside in 1, 2-cis-glycosylation.
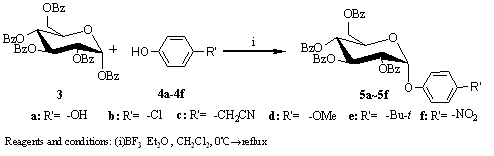
Fig. 2 1, 2-cis-O-glycosylation using penta-O-benzoyl-D-glucoside as donor
As shown in Fig. 2, a series of cis-O-glycosides are synthesized. Under N2, a solution of compound 3 2.5g (3.6mmol) and phenols 4a-4f (7.2mmol) in CH2Cl2 (25ml) are stirred magnetically. The mixture is cooled to 0℃, treated with BF3·Et2O 1.9ml (14.4mmol) then allow the temperature raise to r.t. in 1~2hrs. The mixture is kept under refluxing for another 48hrs then quenched with saturated aq. NaHCO3, diluted with 10ml CH2Cl2 and stirring was continued for 20min, the organic layer is washed by 2×20ml Na2CO3 and 20ml aq. NaCl, dried with anhydr. Na2SO4. The solvent is evaporated under diminished pressure, and the residue is chromatographed by silica gel column (1:6~1:2.5 = ethyl acetate: petroleum ether) to give single isomer or mixture. Yields, a/b ratio and characterizations are listed in Table 1. Without purification, deprotection of 5a to afford α-arbutin 2 is conducted under standard condition with sodium methoxide in methanol. The overall yield of α-arbutin counting from glucose is 66% determined by HPLC using external standard method [8].
Table 1 1H NMRa, EI-MS and melting point dates of compound 5a-5f
Compound |
d (ppm) |
m/z [M+Na]+ |
m. p. (℃) |
Yieldb (%) |
a/b[8] | |||||
H-1 |
H-2 |
H-3 |
H-4 |
H-5 |
H-6a,6b |
|||||
5a |
5.87-5.88 J1,2=3.66 |
5.43-5.47 |
6.35-6.42 |
5.70-5.76 |
4.48-4.60 |
711.11 |
173-174 |
91 |
6.2 |
|
5b |
6.00-6.01 J1,2=3.00 |
5.49-5.51 |
6.39-6.43 |
5.73-5.80 |
4.50-4.58 |
729.13 |
166-167 |
96 |
27.0 |
|
5c |
5.98-6.00 J1,2=3.66 |
5.45-5.50 |
6.36-6.43 |
5.71-5.77 |
4.51-4.54 |
734.02 |
168-170 |
75 |
15.9 |
|
5d |
5.91-5.92 J1,2=2.75 |
5.48-5.50 |
6.40-6.44 |
5.74-5.78 |
4.50-4.66 |
725.11 |
150-152 |
85 |
6.1 |
|
5e |
6.00-6.08 J1,2=3.48 |
5.59-5.64 |
6.51-6.58 |
5.86-5.92 |
4.60-4.78 |
751.05 |
108-110 |
91 |
33.8 |
|
5f |
6.16-6.17 J1,2=3.00 |
5.52-5.54 |
6.39-6.43 |
5.75-5.77 |
4.50-4.56 |
740.11 |
≥195 |
78 |
4.6 |
|
As the substitutions on benzene changing, different yields and anomeric distributions are obtained. p-Nitrophenol and p-methoxyphenol are selected as different glycosyl donors to monitor the anomeric distribution in our glycosylation. The total yields of a and b mixtures (Tab. 1 ) do not vary significantly even after 48 hours. But as the reaction processing (Fig. 3 ) monitored by HPLC, electron donating group provides more a selectivity which is probably due to its quick nucleophilic reaction rate which allows more time for in-situ anomerization. Thus the glycosylation and anomerization may be two processes in sequence but not simultaneous.
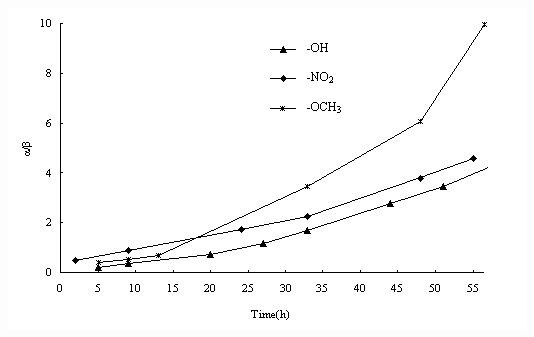
Fig. 3 Anomer distribution with different substituted glycosyl acceptor monitored by HPLC[8]
Acknowledgements
This work was supported by Student Research Training Program (SRTP) of Jiangsu
Province.
REFERENCES
[1] Sugimoto K, Nishimura T, Nomura K, et al. Chem. Pharm. Bull., 2003, 51,798.
[2] Maeda K, Fukuda M, Pharmacol J. Exp. Ther., 1996, 276, 765.
[3] Jacobsson M, Malmberg J, Ellervik U, Carbohydrate Research, 2006, 341, 1266.
[4] Demchenko A V, Synlett., 2003, 9, 1225.
[5] Wang Z X , Shi X X, Chen G R, et al. Carbohydrate Research, 2006, 341, 1945.
[6] France R R, Compton R G, Davis B G, et al. Org. Biomol. Chem., 2004, 2, 2195.
[7] Murakami T, Sato Y, Shibakami M, Carbohydrate Research, 2008, 343, 1297.
[8] HPLC condition: MeOH : H2O=9 : 1, Flow rate: 0.5 ml/min, Column: Arcus C18,
5μm, 4.6*150nm.
方韬,丰巍伟,张明道,方志杰
(南京理工大学化工学院,南京 210094)
摘要 a-熊果苷是一种a-芳苷类络氨酸酶抑制剂。本文报道了一种由五苯甲酰基葡萄糖3作为糖基给体与对苯二酚4a及其衍生物4b-4f在BF3·Et2O催化下进行的糖苷化反应,在简便、温和条件下合成了a-熊果苷中间体5a及其一系列1,2-顺式芳苷衍生物5b-5f,得率分别达到91%和75-96%。对5a脱保护得α-熊果苷,以葡萄糖计算,4步总得率66%。其得率和立体选择性(a/b=6.2)均高于现有方法。5a-5f均为新化合物,用1H-NMR和EI-MS表征了其结构。
关键词 a-熊果苷,五苯甲酰基葡萄糖,1,2-顺式糖苷化,立体选择性合成