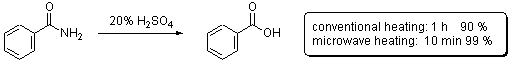
Scheme 1
[E0000]
Microwave-Assisted Organic Synthesis - Back to the Roots
Gabriela Horeis, Sonja Pichler, Alexander Stadler, Walter Gössler, and C. Oliver Kappe*
Institute
of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 28, A-8010
Graz, Austria
Fax: +43-316-3809840
http://www-ang.kfunigraz.ac.at/~kappeco
Received: 29 August 2001 / Uploaded: 30 August 2001
Abstract
This presentation reports the reinvestigation of one of the very first microwave-assisted organic reactions described in the literature, i.e. the hydrolysis of benzamide under acidic conditions. The hydrolysis of benzamide was carried under sealed vessel conditions using microwave irradiation in both dedicated monomode and multimode instruments, with accurate temperature and pressure measurements. Depending on the specified reaction temperature 8-200 fold rate-enhancements were experienced as compared to conventinal thermal reflux conditions. This reactions could be scaled up from 0.5 mL to 50 mL, and also be performed using subcritical water at temperatures of up to 300 °C.
Intoduction
In
1986 Richard Gedye and coworkers published a short communication in Tetrahedron
Letters, entitled "The Use Of Microwave Ovens for Rapid Organic Synthesis"
which for the first time described the utilization and advantages of microwave
irradiation for organic synthesis [1,2]. In this original publication [1]
four different types of reactions were studied, including the hydrolysis
of benzamide to benzoic acid under acidic conditions (Scheme 1). Considerable
rate increases (5 - 1000 fold) were observed for all investigated transformations
when compared to classical thermal reflux conditions. The same year, an
independent study by the groups of Giguere and Majetich describing similar
rate-enhancements in microwave-promoted Diels-Alder, Claisen, and ene reactions
was published in the same journal [3].

Scheme 1
The advantages of using microwave dielectric heating for performing organic reactions were soon realized by many different groups and as a consequence the amount of articles describing high-speed chemical synthesis promoted by microwave irradiation has grown quickly from ~200 in 1995 [4] to ~1000 in 2001. In addition an unusually large number of review articles and commentaries (~60) has been published on this subject covering various aspects of microwave-assisted synthesis [Review database].
Importantly, many of the early microwave-assisted reactions, such as the process shown in Figure 1, were carried out in sealed Teflon or glass vessels using unmodified domestic household ovens [4]. Due to the nature of microwave dielectric heating [5] accurate temperature measurements using conventional means of temperature determination during the irradiation process were not possible at the time. Therefore the reasons for the observed rate-enhancements were in many cases not fully understood and led to a lot of speculation and fierce debate on the existence of so-called non-thermal or specific microwave effects [6,7].
Today, a variety of dedicated microwave reactors for chemical synthesis are commercially available that incorporate built-in magnetic stirring, direct temperature control of the reaction mixture with the aid of fluoroptic probes, shielded thermocouples or IR sensors, and software that enables online temperature/pressure control by regulation of microwave output power (for a list of suppliers, click here).
In this contribution we go back to the initial studies by Gedye and coworkers on the hydrolysis of benzamide (Scheme 1) in order to evaluate the performance and capabilities of different commercially available microwave reactors. In particular, the following issues are addressed:
1) Comparison and switch between mono- and multimode instruments
2) Scale-up characteristics going from a 0.5 mL to a 50 mL scale
3) Potential of using high-temperature microwave reactors for reactions
in subcritical water
Results
and Discussion
A) Small-Scale Benzamide Hydrolysis in Monomode Instruments (Smith Synthesizer, Personal Chemistry)
In order to have accurate data for comparison at hand, we first investigated the hydrolysis of benzamide under classical thermal reflux conditions (~ 100 °C). Experiments were run both with the originally specified 20 % (v/v) sulfuric acid [1], in addition to 5 % (v/v) sulfuric acid. The data so obtained are presented in Figures 1a and b. Thus, complete hydrolysis of benzamide at 100 °C required ca. 60 min in 20 % and 24 h in 5% sulfuric acid.
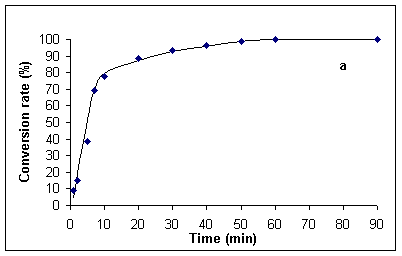
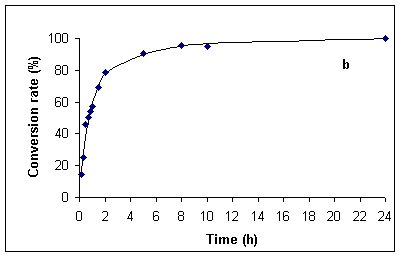
Figure 1. Kinetics for the hydrolysis of benzamide at ca. 100 °C in 20 % (v/v) (Figure a, left) and 5 % (v/v) sulfuric acid (Figure b, right). Hydrolysis rates were determined by HPLC analysis (see Experimental Section).
We next turned our attention to hydrolysis reactions under sealed vessel conditions using heating by microwave irradiation. For these small-scale experiments a monomode instrument with integrated robotics interface for automated use (SmithSynthesizer from Personal Chemistry AB, Figure2a) was employed. In contrast to the original work by Gedye et al. [1], here both direct temperature and pressure measurements are possible (for details, see Experimental Section). After a few optimization runs we discovered that by preselecting 140 °C as reaction temperature, complete conversion to benzoic acid was achieved within 7 min (Table 1). Both the conventional and microwave reaction times therefore nicely agree with the data reported by Gedye in 1986. It is therefore reasonable to assume that a temperature near 140 °C was also reached in Gedye's original experiments involving domestic household microwave ovens [1].
In addition we also carried out microwave-assisted hydrolyis reactions
involving 5 % sulfuric acid. Here, 140 °C reaction temperature proved
to be unsufficient to hydrolyse benzamide within a reasonable time (Table
1). However, increasing the temperature to 180 °C allowed complete
hydrolysis of benzamide within 7 min. Using the unattended, automated processing
features of the SmithSynthesizer, coupled with HPLC analysis, the
optimization results shown in Table 1 were obtainable within a few hours.
Comparing the results of thermal and microwave-assisted hydrolysis rates,
a ca. 8-fold rate-enhancement is calculated for the 140 °C run (20%
sulfuric acid) and a ca. 100-200-fold enhancement for the 180 °C run
(5 % sulfuric acid). Despite the fact that these numbers may seem quite
dramatic, they are not surprising at all if one considers the high reaction
temperatures that are rapidly obtainable in sealed vessels by microwave
heating. Clearly, any evidence for non-thermal microwave effects could
not be observed.
Table 1. Conversion rates for the microwave-assisted hydrolysis of benzamide under different reaction conditions.
|
[min] |
Conversion rate [%] | ||
| 20% H2SO4 (140°C, 5 bar) | 5% H2SO4 (140°C, 4 bar) | 5% H2SO4 (180°C, 11 bar) | |
| 1 | 25 | 9 | 73 |
| 2 | 71 | 31 | 79 |
| 5 | 95 | 51 | 96 |
| 7 | >99 | 64 | >99 |
| 10 | >99 | 83 | >99 |
The above experiments involving microwave-assisted hydrolysis in monomode
instruments involved 2.0 mL of solvent in glass process vials designed
for a filling volume of 2.0-5.0 mL (Figure2b).
In order to cover a variable range of different reaction volumes we have
also carried out experiments in the smaller of the two types of available
process vials, designed for reaction volumes of 0.5-2.0 mL (not shown).
A series of experiments involving hydrolysis of 100 mg/mL of benzamide
in 20 % sulfuric acid was carried out using 0.5, 1.0, 1.5, 2.0, 3.0, 4.0,
and 5.0 mL volume of sulfuric acid. Irradiation in all these cases using
the optimized conditions (140 °C, 7 min) led to quantitative hydrolysis
of benzamide. The maximum pressure, depending on the type of vial and the
volume, was 5-8 bar. Thus, it has been demonstrated that optimized reaction
conditions using the Smith Synthesizer can be employed across the full
range of allowed process volumes, i.e. from 0.5-5.0 mL.
B) Larger Scale Runs in Multimode Reactors (ETHOS Synth Labstation, Milestone).
Another question that is intensely debated in the microwave community is the issue of using multimode versus monomode instruments. In multimode microwave cavities (conceptually similar to a domestic oven), the microwaves that enter the cavity are being reflected by the walls and the load over the typically large cavity. A mode stirrer ensures that the field distribution is as homogeneous as possible. In the much smaller mono- or single-mode cavities, only one mode is present and the electromagnetic irradiation is directed through an accurately designed wave guide onto the reaction vessel mounted in a fixed distance from the radiation source.
We were therefore interested to investigate
if the relatively small-scale reaction conditions that were optimized in
the monomode instrument (see above) could be successfully transformed to
a multimode reactor, capable of processing much larger volumes. In this
context we have carried out the hydrolysis of benzamide in an ETHOS Synth
Labstation, employing a 100 ml sealed PFA reaction vessel in a single high-pressure
HPR 1000 rotor block segment (Figure3). Again,
the use of this instrument allows direct temperature and pressure monitoring,
as well as stirring of the reaction mixture. Using the same concentrations
(100 mg benzamide/mL sulfuric acid) and reaction conditions successful
hydrolysis runs were carried out with 10, 20, and 50 mL volume, i.e. hydrolyzing
up to 5 g of benzamide. Runs were carried out at 140 °C (20 % sulfuric
acid) and 180 °C (5 % sulfuric acid), leading to complete hydrolysis
within 10 min. Although the heating and cooling profiles for the two reactors
were different, these experiments demonstrate that conditions can be sucessfully
taken from one microwave platform to another, going from 0.5 to 50 mL in
scale-up, and from a monomode to a multimode system.
C) Hydrolysis in Near-Critical Water (UltraCLAVE, Milestone).
Chemistry in super-, and subcritical water
(critical point 374 °C, 221 bar) is currently receiving a considerable
amount of attention in the context of the green chemistry debate [8-12].
Since the ionization constant of water reaches a maximum between 200 and
300 °C the concentration of acidic hydronium and basic hydroxide ions
is orders of magnitude greater at e.g. 275 °C (60 bar) than at room
temperature. This effect can be used to run reactions in water at high
temperatures that normally require strong acids [8-12]. The use of microwave
irradiation in synthetic hot-water chemistry has been pioneered by Strauss
and coworkers [13]. Since the hydrolysis of both aromatic and aliphatic
nitriles and amides in super- and subcritical water has been reported in
the literature [14,15], we have attempted to carry out the hydrolysis of
benzamide in such a medium. Since the maximum pressure for the process
vials employed with the Smith Synthesizer is 20 bars, we have initially
attempted to run these hydrolysis reactions in the ETHOS Synth Labstation
with high-pressure segments (Figure 3), where
temperatures of ca. 260 °C (80 bars) can be safely achieved with sealed
Teflon or PFA vessels. Since initial runs at both 220 °C and 250 °C
for 15 min were unsuccessful and only produced reisolated benzamide, we
next switched to a microwave-heated high-temperature autoclave system (UltraCLAVE
from Milestone, Inc., Figure 4) that allows operation
under microwave irradiation of up to 300 °C and 200 bar pressure. Here
indeed hydrolysis of benzamide to benzoic acid was observed at 270 °C
(ca. 25% conversion after 60 min), and at 300 °C (ca. 75 % conversion
after 30 min). However, the yield of reisolated material was generally
low (ca. 20-40 %), presumably due to concomittant decomposition of benzoic
acid and benzamide involving other pathways [14,15]. Work along these lines
is continuing in our laboratories.
Experimental Section
A) Monitoring Benzamide Hydrolysis:
As benzamide and benzoic acid are virtually insoluble in 4 °C water and sulfuric acid, the reaction products could be simply isolated by suction after standing for several hours in a refrigerator and washed with cold water. For all kinetic investigations described herein a HPLC method was developed based on the analyis of reprecipitated material after cooling (>95% recovery). After drying, equivalent amounts of material were dissolved in a minimum amount of acetonitrile and filled up to 10 mL with water. That solution was injected into the HPLC (HP Series 1050, Column LiChrospher 100 RP-18 (5µm) 119 mm x 3 mm, E. Merck, Darmstadt, Germany), using a gradient of [acetonitrile/water 5:95]/[acetonitrile] 7:3 (0-2 min) and acetonitril (2-10 min) as the mobile solvent. The eluation resulted in a retention time of 2.5 min for benzamide and 3.7 min for benzoic acid. For reference purposes some samples were also worked-up according to the method described by Gedye et al. [2] involving extraction methods. In our hands both methods gave virtually identical results.
B) Microwave Reactors:
SmithSynthesizer™
(Personal Chemistry AB) (see Figure 2a).
The system operates at a frequency of
2.45 GHz with continuous microwave irradiation power from 0-300 W.
The reaction vials are glass-based ca. 10 mL tubes, sealed with teflon
septa and an aluminium crimp top. Vials in 0.5-2.0 and 2.0-5.0 mL scale
are available, both equipped with appropriate stirring bars. The process
vials are moved in an automated fashion by a gripper incorporated to the
platform. Inside the cavity, applying the pressure sensor as additional
seal, the vials can be exposed to 20 bars and 250°C. Temperature is
measured by infrared thermometry on the outer surface of the process vial.
The microwave output power is regulated by the software algorithm, so that
the preselected maximum temperature is maintained for the desired reaction
time. Reagents can either be filled manually into the vials before capping,
or can be dispensed through the teflon septuum via the liquid handler incorporated
into the platform. As benzamide is insoluble in sulfuric acid at ambient
temperature, the experiments are performed as "single runs", specifying
the time and temperature each process vial from a designated rack position.
After the irradiation period the reaction vessel is cooled down rapidly
(approx. 60 seconds) to ambient temperature by compressed air (gas jet
cooling). Dedicated software (Smith Workflow Manager) integrated with ISIS/Draw
supports the entire process from planning and preparation to instrument
control and results documentation.
ETHOS
Synth Labstation (Milestone Inc.) (see Figure
3).
The multimode microwave reactor has a
twin magnetron (2 x 800W, 2455 MHz) with a maximum delivered power of 1000W
in 10W increments (pulsed irradiation). A rotating microwave diffuser ensures
homogeneous microwave distribution throughout the plasma coated PTFE cavity
(35 cm x 35 cm x 35 cm). For the experiments carried out in sealed vessels
a 100 mL PFA reaction vessel contained in a single high-pressure HPR1000
rotor block segment was employed. Built-in magnetic stirring (teflon-coated
stirring bar) was used in all operations. During experiments, time, temperature,
pressure, and power was monitored/controlled with the "easyWAVE" software
package (Vers. 3.2.). Temperature was monitored with the aid of a fluoroptic
probe (ATC-FO) and/or with a shielded thermocouple (ATC-300) inserted directly
into the corresponding reaction container. For experiments in sealed vessels
a pressure sensor (APC-55) was additionally employed.
UltraCLAVE
Microwave Autoclave (Milestone Inc.) (see Figure
4).
The ultraCLAVE combines microwave heating
with high pressure vessel technology for extraordinary performance capabilities.
Reactions can be conducted at pressures and temperatures up to 200 bar
and 350°C. This is a microwave heated autoclave developed for parallel
reactions, mainly for digestion in analytical chemistry. It is equipped
with a rotary holder system, suitable for 31 10 mL quartz reaction vials.
The holder is surrounded by a water filled teflon bomb and a starting pressure
can be put on the reaction vessels manually before starting the irradiation.
After reaction a cooling period of about 45 min (depending on reaction
temperature) is required. Several of the 10 ml quartz glass vials were
filled with 300 mg benzamide and 5 ml water and were placed in the corresponding
vial holder. After fixing the holder in the MLS UltraCLAVE, the cavity
was closed and initial pressure of 70 bar (nitrogen) was applied. The reaction
mixture was irradiated at 300°C for 30 min or at 270°C for 60 min,
respectively, leading to pressures of approx. 140 bar, subsequently followed
by a 60 min cooling period.
Conclusions and Outlook
In conclusion we have reinvestigated the original work by Gedye and coworkers on the microwave-assisted hydrolysis of benzamide in sulfuric acid. Using dedicated microwave instruments this reaction could be performed safely under a variety of different reaction conditions employing three different microwave reactors. Particularly noteworthy is the fact that no difference between a monomode and a multimode reactor was observed with this chemistry, and that it was possible to perform these reactions from 0.5 mL to 50 mL scale using identical reaction conditions.
Microwave-assisted synthesis in general
is likely to have a large impact on synthetic organic chemistry, in particular
the medicinal/combinatorial chemistry communities. Compared to traditional
processing of organic synthesis, microwave-enhanced chemistry saves significant
time and very often improves yields. It has also been demonstrated in a
number of examples that previously practically impossible transformations
are successfully completed using microwave irradiation. The short reaction
times open up new approaches for rapid testing of ideas and fast iterations
in protocol development. While microwave heating is today still considered
by some as a laboratory curiosity, we believe that this technology will
be used extensively in the future for many chemical processes requiring
heat.
Acknowledgements
We wish to thank PersonalChemistry AB, Uppsala
for use of the SmithSynthesizer. We also wish to thank G. A. Strohmeier
for assisting in HPLC measurements.
References and Notes
[1] Gedye R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L. Tetrahedron Lett., 1986, 27, 279-282.