[A0039]
Phthalides and Chromones from 4-Coumarinacetic acids, Study of Beneficial Effects of Microwave Irradiation on Synthesis.
Margita Lacova1, Dusan Loos2, Jarmila Chovancova1, Katarina Kralova2
1Department of Organic Chemistry and 2Institute of Chemistry, Faculty of Natural Sciences, Comenius University, Mlynska dolina CH-2, SK-842 15 Bratislava, Slovakia
; Tel.: +421 7 60296338; Fax: +421 7 60296690;Received: 20 August 1999 / Uploaded: 26 August 1999
Abstract
3-(4-Coumarinylmethylene)phthalides and 2-, or 3-(4-coumarinylethenyl)-4-chromones were prepared by condensation reaction. Beneficial effect of microwave irradiation on length of reaction time was investigated. Some subsequent reactions of these derivatives with nucleophiles are described. Nucleophile attack is studied by AM1 theoretical method.
Keywords :
3-formylchromones, dicarboxylic acid anhydrides, condensation reaction, pyridine derivatives
Introduction
Phthalides, coumarines and chromones are well frequented in the literature, since they use to be utilised as reactive intermediates. Some of them are, moreover, highly biologically active compounds e. g. pharmaceuticals [1], bactericides [2], pesticides [3] and materials for cosmetics [4 - 5]. Phthalide, coumarine and chromone derivatives are highly versatile molecules because their reactivity towards nucleophiles provides useful route to preparation of a variety rearranged products and new heterocyclic systems.
This paper extended our previous work on aldol condensation with R-acetic acids as e.g. 2-furylacetic [6], 2-, or 3-thienylacetic [7, 8], 3-indolylacetic, 2-benzothiazolylthio [9, 10], or 2-benzoimidazolylthioacetic, aryloxy- and arylthioacetic acids [11].This work was aimed to prepare coumarine systems bonding phthalide or chromone groups at position 4 of coumarine ring.
The possibility to use of 4-coumarinacetic acids as easily decarboxylation compounds for Perkin condensation of phthalides and chromones was investigated. The dependence of yields upon reaction conditions as temperature, time and microwave irradiation was also studied.
Further we oriented our study to theoretical calculation of a heat of formation for the transition state and molecular electrostatic potential for grand state. The additional aim of this study was to investigate the inhibition of photosynthetic electron transport in the algal suspension of Chlorela vulgaris and spinach chloroplasts in order to determinated the site of action of prepared compounds in the photosynthetic apparatus.
Results and Discussion
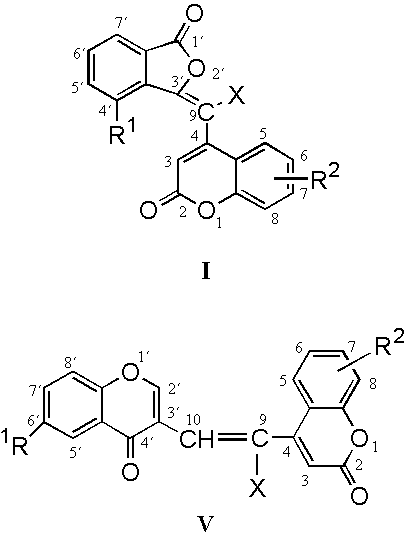
By condensation reaction under both classic and irradiation conditions we attempted to prepare following compounds and their derivatives:

On the basis of the experimental results we conclude that 4-coumarine acetic acids have very reactive methylene group, but they are less stable, they very easily decarboxylate by heating. From this reason the low yield 20 - 25 % of phthalides I (Scheme 1) was not surprising. This step involved free solution conditions and 180 - 190 °C high temperature. 4-Methyl-coumarines were isolated as by - products.
On the other hand the reaction mixture which is containing 3-NO2 phthalanhydride turned brown quickly at temperatures higher than 150 °C, then no products of phthalide were obtained.
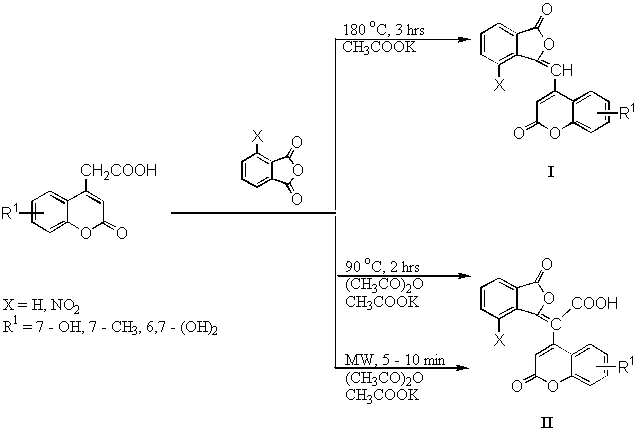
Yield I classic X = H 20 - 25 % MW X = H 0 %
X = NO2 0 % X = NO2 0 %
Yield II classic X = H 0 % MW X = H 50 %
X = NO2 65 - 75 % X = NO2 60 %
Scheme 1
The next step is modification of the procedure using acetic anhydride. It is
advantageously to use for the synthesis of nitrophthalides II without
decarboxylation. It is important that again no condensation products were obtained from
unsubstituted phthalanhydride, except 4-methylcoumarine derivatives. By our experimental
results is evident beneficial effect of microwave irradiation on condensation reaction of
4-coumarinacetic acids. They very well reacted, without decomposition, with carbonyl
groups of phthalanhydrides and 3-, or 2- formylchromones for 4 - 10 minutes. 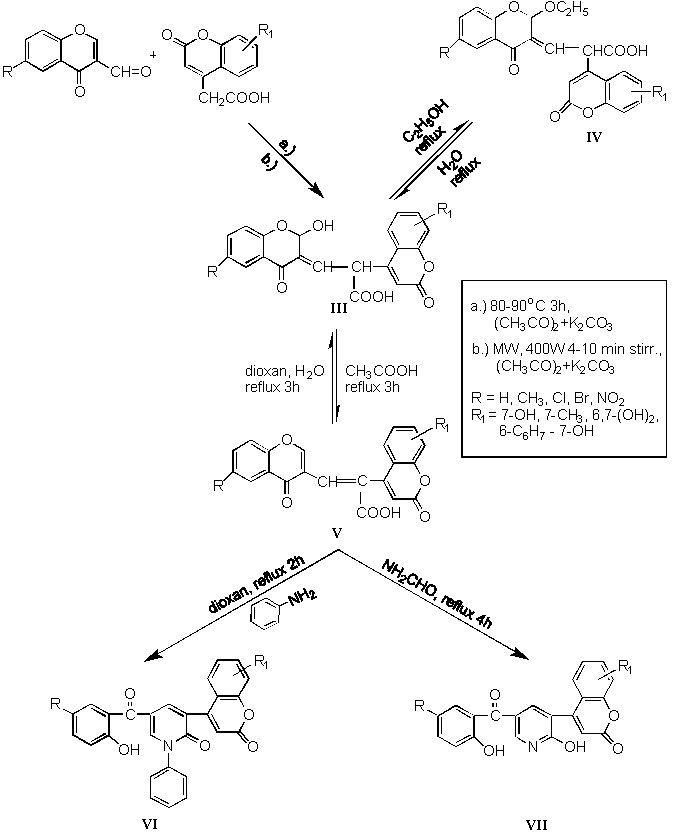
Scheme 2
The microwave experiments included homogenous conditions. During microwave heating the solid components were dissolved in acetic anhydride.
In condensation reaction of 3-formylchromone with 4-coumarinacetic acids we expected to obtain chromone derivatives V. This reaction of both compounds led to arising of 2-hydroxy chromone derivatives III. Compound V were formed only by refluxing of compounds III or IV in acetic acid. Compounds III, IV and V were able to change into each other as is indicated in Scheme 2. By experimental results we proved that only chromone part of compounds III, IV and V reacted with primary amine or formamide on position 2´. After nucleophilic attack the pyrone ring is opening and then is capable to cause the rearrangement of chromone system into pyridine derivatives VI and VII.
2-Formylchromones were more reactive as 3-formyl analogues. Condensations with former compounds were very successful at 25 - 30 ° C with excellent yields (90 - 95 %) of products VIII (Scheme 3).
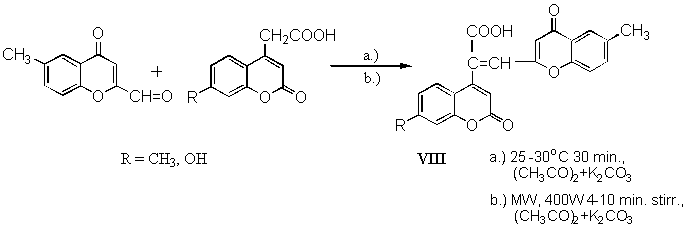
Scheme 3
Conclusion
The optimal structures, heat of formation and charge densities for compound V were calculated by quantum chemistry AM1 method [ 1 2 ] to study the nucleophilic addition. Charge densities were used to obtain molecular electrostatic potential [ 1 3 ] . Three centres of the nucleophile attack were selected in position 2, 2´ and 4´. For these positions three transitions states were calculated. Activation energy is more favourable for attack in position 2´ than in position 2 (difference is 125 kJ mol-1) and position 4´ (difference is 70 kJ mol-1). The same results it can be shown by the distribution of the molecular electrostatic potential on the Van der Waals surface of the molecule V (Fig. 1).
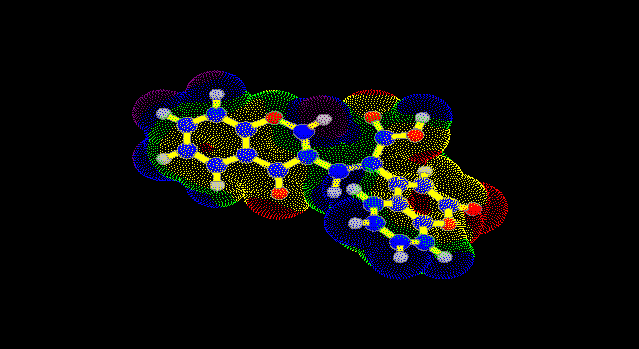
Fig. 1
. Molecular electrostatic potential of the compound V (R = H, R1 = H).(The most positive values are in blue colour and the most negative values are in red colour)
The highest values of molecular electrostatic potential (positive values) are in blue colour and the smallest value is in red colour (negative values). Positions 2 and 4´ are in area with negative values and position 2´ is in area with positive value of molecular electrostatic potential.
On the base of the experimental and theoretical results we can assume that the nucleophilic attack is going to position 2´.
In this paper our investigations led to knowledge that 4-coumarineacetic acids are suitable components for condensation reactions with formylchromones and phthalic anhydrides under both classic and microwave heating conditions.
Experimental section
General
The melting points were determined with a Kofler block.
1
H NMR (300 MHz) spectra were measured on a spectrometer Varian Geminy 2000 in solution of deuterated CHCl3 or DMSO with TMS as internal standard.All microwave assisted reactions were carried out in a Lavis - 1000 multi Quant microwave oven. The apparatus has been adapted for laboratory application with an external reflux condenser. The reaction course was monitored by thin - layer chromatography in ethyl acetate - isohexane.
The 1H NMR - spectra of the prepared compounds were corresponding to their structures.
Elemental analyses for C, H, N were within ±0.3 % of theoretical values.
The IR - measurement was recorded in nujol on a Specord IR-75 spectrometer
The theoretical data have been obtained by the AM1 quantum chemical method with standard parametrization (keyword PRECISE) and optimalization all geometric parameters.
Chemical part
Phtalides Ia - Ie
4-Coumarinacetic acid (2 g) was added in small doses during 10 minutes under stirring to a mixture of freshly remelted phthalic anhydride (2 g) and potassium acetate (0.01 g) at 190 °C. The reaction was allowed to continue for 1 h. The still warm mixture was poured into water solution of NaHCO3. Insoluble yellow part was separated and crystallized from acetic acid or nitrobenzene.
Ia 3-(6-Methyl-4-coumarinylmethylene) phthalide (M = 304.3)
M.p. : 332 °C IR (cm-1) n (CO)coum.a 1698 n (CO)phth.b 1798
Ib 3-(7-Methyl-4-coumarinylmethylene) phthalide (M = 304.3)
M.p. : 349 °C IR (cm-1) n (CO)coum. 1693 n (CO)phth. 1789
Ic 3-(6, 7-Dimethyl-4-coumarinylmethylene) phthalide (M = 318.3)
M.p. : 353 °C IR (cm-1) n (CO)coum. 1696 n (CO)phth. 1787
Id 3-(5, 7-Dimethyl-4-coumarinylmethylene) phthalide (M = 318.3)
M.p. : 284 - 286 °C IR (cm-1) n (CO)coum. 1700 n (CO)phth. 1794
Ie 3-(7-Hydroxy-4-coumarinylmethylene) phthalide (M = 318.3)
M.p. : 351 - 353 °C IR (cm-1) n (CO)coum. 1694 n (CO)phth. 1781
(acoum. - coumarin, bphth. - phthalide)
Phtalides IIa, IIb
Method A (classic)
A mixture of 4-coumarinacetic acid (2 g), 3-nitrophthalanhydride (2g), acetic acid anhydride (10 cm3) and K2CO3 (0.01 g) was stirring at 90 for 2 hrs. After cooled the solid products were separated, washed with acetone and recrystallized from acetic acid. Yields about 60 - 68 %.
IIa 7-Methyl-4-(4-nitro-1H,3H-1-oxo-3, 3-isobenzofurandiyl) coumarineacetic acid
M = 349.3 M.p. 292 - 294 (decomp.)
IR (cm-1) : n (OH) 3090 n (CO)coum. 1680 n (CO)acid 1715 n (CO)phth. 1805 n s(NO2) 1342
n
as(NO2) 1530IIb 7-Hydroxy-4-(4-nitro-1H, 3H-1-oxo-3, 3-isobenzofurandiyl) coumarinacetic acid
M = 351.3 M.p. 243 - 246 °C
IR (cm-1) : n (OH) 3480, 3100 n (CO)coum. 1675 n (CO)acid 1710 n(CO)phth. 1808 n s(NO2) 1340
n
as(NO2) 1530
Method B (microwave irradiation)
The same mixture as used in the procedure A was irradiated in microwave oven at 400 W for 10 minutes. The isolation procedure is the same as above.
Chromones IIIa, IIIb
Method A (classic)
A mixture of 4-coumarinacetic acid (2 g), 3-formylchromone (2.2 g) (or 2-formylchromone), acetic acid anhydride (10 cm3) K2CO3 (0.01 g) was stirring at 90 °C for 1 h. After cooled the mixture was poured into 50 ml of water. The solid products was separated and recrystallized from acetone. Yields 60 - 65 %.
Method B
The same mixture as was used above (A - method) was irradiated in microwave oven at 400 W for 5 - 10 minutes. The isolation of products is the same. Yields 64 %.
IIIa 7, 8-Diacetyloxy-4-(6-chloro-4-oxo-2-hydroxybenzopyran-3, 3-diyl)coumarinacetic acid
M = 528.5 M.p. = 196 - 198 ° C
1H NMR (DMSO), d ppm : Some of characteristic signals for compound:
2.351 (3H, s, CH3), 2.437 (3H, s, CH3), 6.48 (1H, d, 3J = 6.04 Hz, H-8),
7.57 (1H, d, 3J = 6.04 Hz, H-9), 6.66 (1H, d, 4J = 3.59 Hz, H-2´), 6.66 (1H, d, 4J = 3.57 Hz, OH)
Chromones IVa - IVb
Compound III were refluxed in ethanol for 6 h and crystallized from the ethanol. Yields 88 %.
IVa 7, 8-Diacetyloxy-4-(6-chloro-4-oxo-2-ethyloxybenzopyran-3, 3-diyl)coumarinacetic acid
M = 540.5 M.p. = 232 - 235 ° C
1H NMR (DMSO), d ppm : Some of characteristic signals for compound:
1.21, 1.23, 1.25 (3H, t, CH3), 2.34 (3H, s, CH3), 2.42 (3H, s, CH3), 3.79 (2H, q, CH2),
6.07 (1H, s, H-2´), 6.49 (1H, d, 3J = 6.20 Hz, H-8), 7.41 (1H, d, 3J = 6.2Hz, H-9),
7.88, 7.87 (1H, d, H-5´)
IVb 7-Acetyloxy-4-(6-nitro-4-oxo-2-ethyloxybenzopyran-3, 3-diyl)coumarinacetic acid
M = 509.4 M.p. = 218 - 220 ° C
1H NMR (DMSO), d ppm : Some of characteristic signals for compound:
1.13, 1.15, 1.17 (3H, t, CH3), 2.32 (3H, s, CH3), 3.85 (2H, q, CH2), 6.52 (1H, s, H-2´),
6.62 (1H, d, 3J = 6.00 Hz, H-8), 7.36 (1H, d, 3J = 6.00 Hz, H-9), 8.49 (1H, d, 4J = 2.20 Hz, H-5´)
Chromones Va - Vb
Chromones III and IV were in acetic acid dissolved and heated on reflux for 3h. After cooled solid products were separated. Yields 78 %.
Va 7, 8-Diacetyloxy-4-(6-chloro-4H-4-oxo-benzopyran-3-ylmethylene)coumarinacetic acid
M = 510.0 M.p. = 221 - 223 ° C
1H NMR (DMSO), d ppm : Some of characteristic signals for compound:
2.36 (3H, s, CH3), 2.43 (3H, s, CH3), 6.74 (1H, s, H-3), 8.09 (1H, d, 4J = 2.2
Hz, H-5´), 8.874 (1H, s, H-2´)
Vb 7-Methyl-4-(6-nitro-4H-4-oxo-benzopyran-3-ylmethylene)coumarinacetic acid
M =419.0 M.p. = 206 - 208 ° C
1H NMR (DMSO), d ppm : Some of characteristic signals for compound:
2.43 (3H, s, CH3), 6.54 (1H, s, H-3), 7.95 (1H, s, H-9), 8.34 (1H, d, 4J = 2.74
Hz, H-5´), 8.49 (1H, d, 4J = 2.7 Hz, H-2´),
Vc 7-Methyl-4-(4H-4-oxo-benzopyran-3-ylmethylene)coumarinacetic acid
M = 373.2 M.p. = 261 - 263 ° C
1H NMR (DMSO), d ppm : Some of characteristic signals for compound:
2.46 (3H, s, CH3), 6.43 (1H, s, H-3), 7.93, 7.96 (1H, d, H-2)
N-phenyl-2-oxo-1H, 2H-pyridine derivative VI
Dioxan solution of chromone Vc (2 g) and aniline (1.4 g) was heated on reflux for 2 h. After precipitated the product was separated and crystallized from mixture of dimethyl sulfoxide - ethylacetate. Yield 45 %. M.p. 214 - 215 ° C.
Pyridine derivatives VII
Chromone Vc (2 g) was heated 4 h on reflux in formamide. After cooling the product was filtered off and recrystallized from mixture dimethyl sulfoxide - ethanol. Yield 52 %. M.p. 342 - 343 ° C.
Chromones VIIIa and VIIIb
Method A (classic)
A mixture of 4-coumarin acetic aci (0.2 g), 2-formylchromone (0.22 g) acetic acid anhydride (5 cm3) K2CO3 (0.1 g) was stirring at 30 ° C for 30 minutes. Solid compound were separated and crystalized from chloroform. Yield 90 %.
Method B
The same mixture as was used above was irradiated in microwave oven at 400 W for 4 minutes. The isolation of products is the same. Yields 85 %.
VIIIa 7-Methyl-4-(6-methyl-4H-4-oxo-benzopyran-2-ylmethylene) coumarin acetic acid
M = 388.4 C13H16O6 M.p. = 293 - 294 ° C
1H NMR (CDCl3)a : 2.48 (6H, s, CH3), 6.43 (1H, s), 6.56 (1H,s), 6.98, 6.93 (1H, d),
7.22, 7.19, 7.17 (2H, t), 7.49, 7.46 (1H, d), 7.57, 7.54 (1H, d), 7.68,
7.65 (1H, s), 7.84, 7.79 (1H, d), 8.01 (1H, s, COOH)
VIIIb 7-Acetyloxyl-4-(6-methyl-4H-4-oxo-benzopyran-2-ylmethylene) coumarin acetic acid
M = 432.4 C24H16O8 M.p. = 251 - 254 ° C
1H NMR (CDCl3)a : 2.36 (3H, s, CH3Ar ), 2.48 (3H, s,CH3CO), 6.43 (1H,s), 6.599,
6.597 (1H, d), 6.99, 6.94, (1H, d), 7.16 - 7.14 (1H, q, 3J = 8.6 Hz,
4J = 2.2 Hz), 7.21, 7.21 (1H, d, 4J = 2.2 Hz), 7.49, 7.46 (1H, d, 3J =
8.8 Hz), 7.57 - 7.54 (1H, q, 4J = 2.2 Hz), 7.76, 7.78 (1H, d, 3J = 8.0
Hz), 7.81 (1H, s), 8.007, 8.010 (1H, d, COOH)
(a in ppm)
Acknowledgements
: Our thanks are due to Dr. E.Solcaniova and Dr. K. Gaplovska for 1H NMR spectra and elemental analysis (Institute of Chemistry, Faculty of Natural Sciences, Comenius University, Bratislava).Financial support for this research by the Slovak Grant Agrncy (grant No. 1/5085/98) gratefully acknowledged.
References
1. Nohara, A.; Umetani, T.; Sanno, Y.; Tetrahedron 1974, 30, 3553.
2. Lacova, M.; Stankovicova, H.; Odlerova, Z.; Il Farmaco 1995, 50, 885.
3. Lacova, M.; Chovancova, J.; Konecny, V.; Chem. Papers 1986, 40, 121.
4. Vaugham, C. D.; J, Soc. Cosmet. Chem. 1985, 33, 319.
5. Conner, D. E.; Ger. Offen 3.002304; Chem. Abstr. 1981, 94, 36119.
6. Lacova, M.; Perjessy, A.; Hrnciar, P.; Chem. Zvesti 1969, 23, 53.
7. Lacova, M.; Hrnciar, P.; Chem. Papers 1985, 39, 135.
8. Lacova, M.; Chovancova, J.; Veverkova, E.; Toma, S.; Tetrahedron 1996, 52, 14995.
9. Lacova, M.; Chem. Zvesti 1969, 23, 450.
10. Lacova, M.; Chem. Papers 1986, 40, 95.
11. Lacova, M.; Chem. Zvesti 1973, 27, 525.
12. Dewar, M. J. S.; Zoebisch, E.; Healy, E.; Stewart, J. P. P.; J. Am. Chem. Soc. 1985, 107, 3902.
13. Ertl, P.; Chem. Listy 1992, 86, 465.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0039 as the message subject of your e-mail.