Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0018]
Polymalonates derived from
ortho-bromomethylated tetraarylporphyrins
Norbert Jux*
Institut fur Organische Chemie der Universitat Erlangen-Nurnberg, Henkestr. 42, 91054 Erlangen
E-mail: [email protected]
Received: 8 July 2000 / Uploaded: 29 July 2000
Meso-tetraphenylporphyrin (TPP) and its derivatives are commonly used for the construction of photosynthetic model compounds,1 enzyme mimetics, and artificial receptors.2 Also, with regard to materials sciences, TPPs are found for example in dendrimers,3 as liquid crystalline materials with discotic behavior,4 and as part of optoelectronic devices.5,6 Since the derivatization of TPPs has been well studied, it is possible to tailor their properties allowing adaption into the desired specific environment. Obviously, novel materials containing porphyrins have become a major research field in porphyrin chemistry. It appears to us that by using the inherent geometry of porphyrins interesting new conjugates would be available. In this paper we wish to introduce a series of polymalonates derived from bromomethylated tetraarylporphyrins, which are useful precursors for nucleophilic substitutions.
Because tetraphenylporphyrins are easy to prepare and are well studied we decided to use these compounds as foundation for our research. Our aim was to generate porphyrins with easily modifiable groups in close proximity to the core. Therefore, these substituents had to be placed at the ortho-positions of the phenyl rings. TPPs with ortho-phenyl substituents are subject to extensive research7 because ortho-groups prevent aggregation and protect the core of the porphyrin from side reactions. The modification of some derivatives, especially of amino- and hydroxyderivatives, was extensively studied and led to beautiful molecules like cyclam-capped porphyrins8 or barrel-shaped systems9 with a porphyrin center. Molecular modeling suggests that the geometric situation of substituents in the ortho- positions should lead to better interactions with the porphyrin core. Also, due to the size of the porphyrin the rotation of such groups will be strongly hindered.
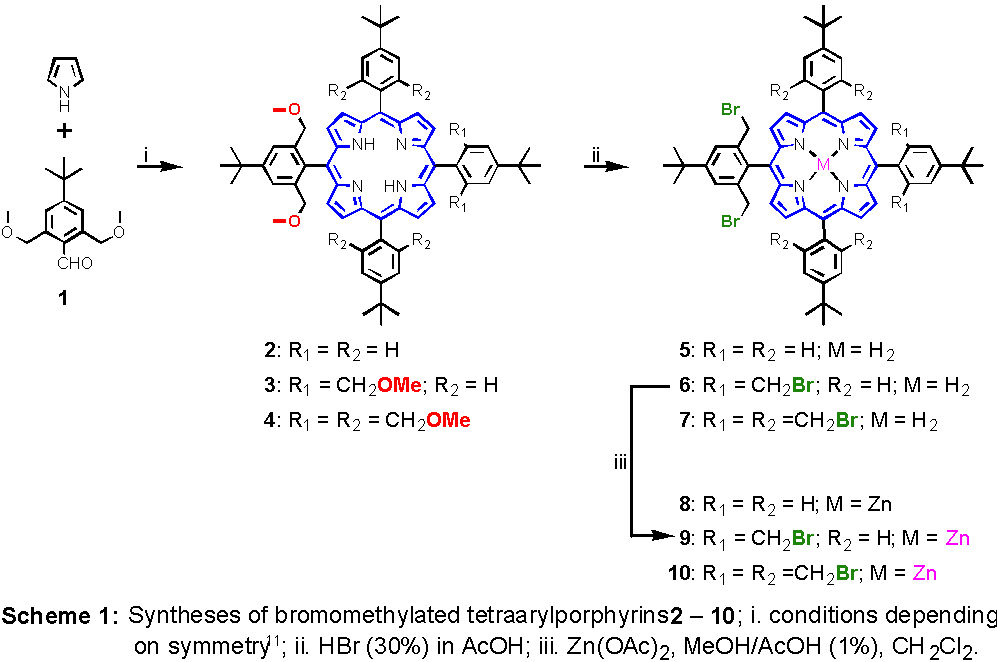
We recently reported on the synthesis of two-, four-, and eightfold ortho-bromomethyl substituted zinc-tetraarylporphyrins, 8, 9, and 10, respectively.10 These compounds were available by applying standard porphyrin synthetic methodology to 2,6-bis(methoxymethyl)-4-t-butylbenzaldehyde
1. The resulting methoxymethylated porphyrins 2, 3, and 4, were transformed into the corresponding bromomethyl compounds 5, 6, and 7, by treatment with HBr in acetic acid. Chelation of Zn2+ was accomplished by addition of zink acetate in methanol/1%acetic acid to a solution of a porphyrin in methylene chloride (Scheme 1). Nucleophilic substitution reaction of the reactive bromomethyl groups with various nucleophiles such as thiolates, cyanide, azide, and pyridine are easy to perform. Here, we wish to report on the reactions of zink porphyrins 8 and 9 with sodium diethyl malonate.

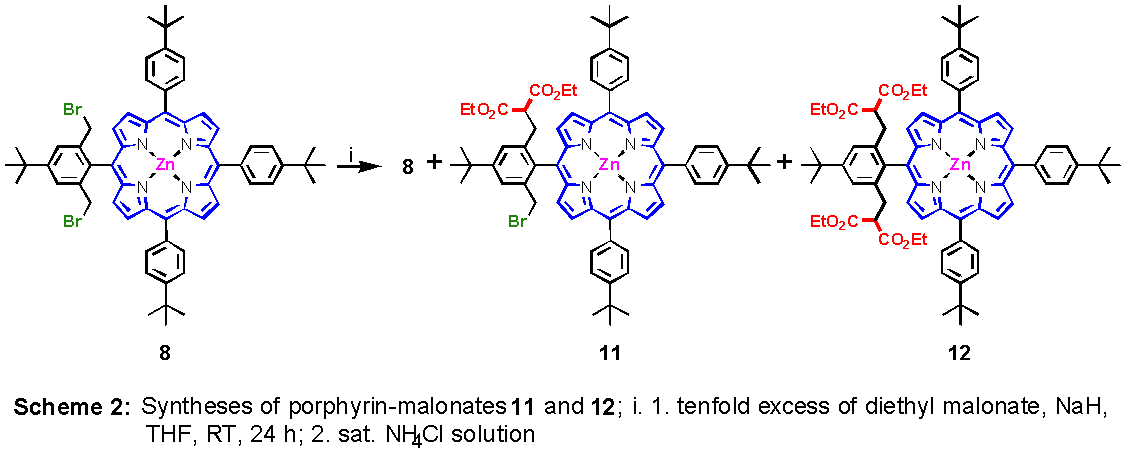
The reaction of 8 and 9 with diethyl malonate using sodium hydride as base in THF proceeds in a smooth way to produce a variety of porphyrinic malonates. Although the reaction can be brought to completion by using a large excess of malonate and higher temperatures, we thought the intermediates to be of interest, too. We did not consider 10 as a suitable candidate for this procedure because the number of possible isomers is to large.12
When the bis(bromomethyl) compound 8 was treated with a tenfold excess of malonate anion, a mixture of starting material 8, mono- and bismalonate 11 and 12, respectively, were obtained after 24 hours at room temperature, when the reaction was quenched with ammonium chloride solution (Scheme 2). The porphyrins were separated by column chromatography using silica gel and a mixture of methylene chloride/hexanes 1:1 as eluent. As was expected, unreacted 8 eluted first, followed by the more polar monomalonate 11. Bismalonate 12 as the most polar compound in the mixture came last. The 1H NMR and mass spectra are in agreement with the assumed structures of the substituted porphyrins. The C2v-symmetry of 12 is nicely reflected in its 1H NMR-spectrum.
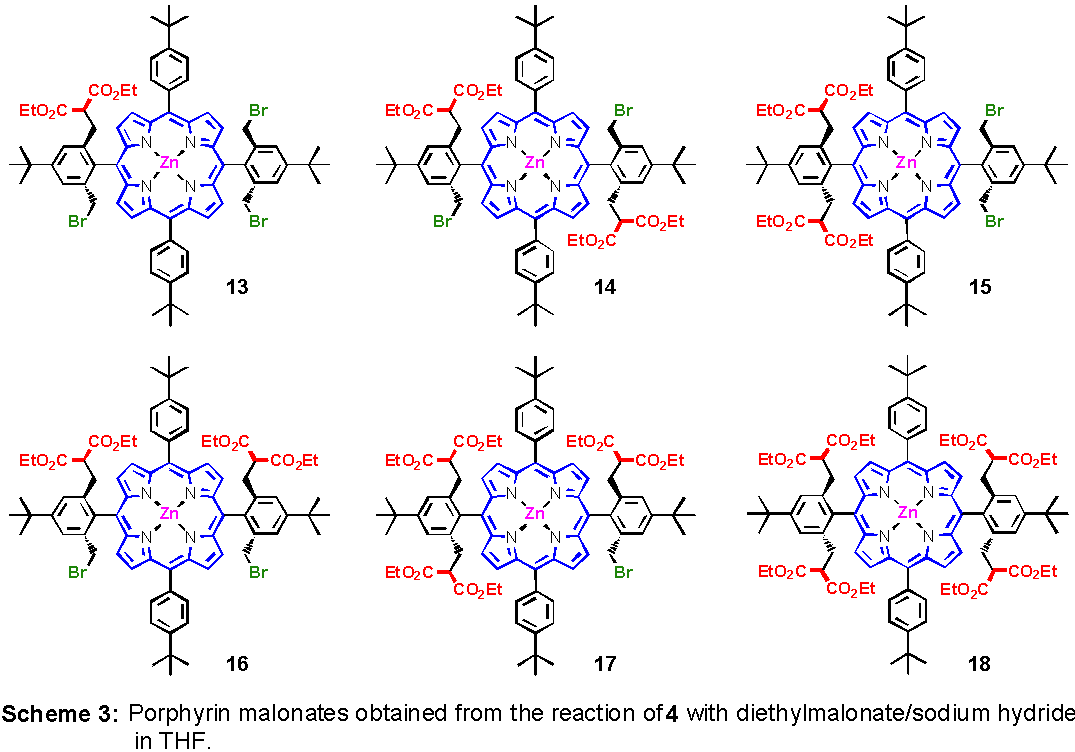
The reaction of the tetrabrominated porphyrin 9 with a moderate excess of sodium malonate in THF at room temperature gave the porphyrin-monomalonate 13, three difunctionalized isomers 14 - 16, one tri- and one tetra-substituted 17 and 18, respectively. Although seven different porphyrins are present in the reaction mixture, the separation using column chromatography on silica gel proved to be possible. The eluation started with methylene chloride/hexanes 1:1. After the starting material 3 was eluted the amount of methylene chloride was continually encreased. The first substituted porphyrin found was the monomalonate-tribromoporphyrin 13. The next isolated compound was the bismalono-conjugate with C2h-symmetry 14 (anti-substitution of the malonate groups). Very close in polarity was the C2v-symmetrical porphyrin 15 with both malonates attached to the same phenyl group. The last possible bismalonate 16 showed a higher polarity due to the syn-position of the remaining bromine atoms (C2v-symmetry). The trisubstitued porphyrin 17 was much more polar and was eluted only with pure methylene chloride. Finally, tetramalonate 18 was isolated after addition of 1% of ethyl acetate to the eluent. The degrees of substitution, the symmetries and the substitution pattern were identified by mass and 1H NMR spectroscopy (Scheme3).
Compounds 11 � 18 behave spectroscopically like other comparable zinc tetraarylporphyrins. They show no unusual features in the UV/Vis spectra indicating an only small influence of the malonate groups on the porphyrin chromophor.
First attempts to further modify the bis(malonate) 12 look promising. The di-anion was generated using the same conditions, i.e. sodium hydride in THF, and quenched with ethyl bromide. Mass spectroscopy reveals the clean formation of a mone- and diethylated species. We plan to extend these reactions to chiral halides. Also, the saponification of the ester groups will be studied. Porphyrins 11 and 13 � 17, which have unreacted bromomethyl groups available can be modified as has been shown with other compounds.10 This possibility allows for the construction of differentially substituted porphyrins with even further ways of modification.
References
(1) Osuka, A.; Marumo, S.; Mataga, N.; Taniguchi, S.; Okada, T.; Yamazaki, I.; Nishimura, Y.; Ohno, T.; Nozaki, K. J. Am. Chem. Soc. 1996, 118, 155 -168; Steinberg-Yfrach, G.; Liddell, P. A.; Hung, S.-C.; Moore, A. L.; Gust, D.; Moore, T. A. Nature, 1997, 385, 239 - 241; Steinberg-Yfrach, G.; Rigaud, J.-L.; Durantini, E. N.; Moore, A. L.; Gust, D.; Moore, T. A. Nature, 1998, 392, 479 - 432; Liddell, P. A.; Kuciauskas, D.; Sumida, J. P.; Nash, B.; Nguyen, D.; Moore, A. L.; Moore, T. A.; Gust, D. J. Am. Chem. Soc. 1997, 119, 1400 - 1405; Arimura, T.; Brown, C. T.; Springs, S. L.; Sessler, J. L. Chem. Commun. 1996, 2293 - 2294; Flamigni, L.; Barigelletti, F.; Armaroli, N.; Collin, J.-P.; Sauvage, J.-P.; Williams, J. A. G. Chem. Eur. J. 1998, 4, 1744 -1754.
(2) Collman, J. Inorg. Chem. 1997, 36, 5145 - 5155; French, R. R.; Wirz, J.; Woggon, W.-D.; Helv. Chim. Acta 1998, 81, 1521 - 1527; Wagenknecht, H.-A.; Claude; Woggon, W.-D. Helv. Chim. Acta 1998, 81, 1506 - 1520; Mizutani, T.; Ema, T.; Tomita, T.; Kuroda, Y.; Ogoshi, H. J. Am. Chem. Soc. 1994, 116, 4240 - 4250; Ogoshi, H.; Mizutani, T. Acc. Chem. Res. 1998, 31, 81 - 89; Rose, E.; Soleilhavoup, M.; Christ-Tommasino, L.; Moreau, G.; Collman, J. P.; Quelquejeu, M.; Straumanis, A. J. Org. Chem. 1998, 63, 2042 - 2044; Furusho, Y.; Kimura, T.; Mizuno, Y.; Aida, T. J. Am. Chem. Soc. 1997, 119, 5267 - 5268; Takeuchi, M.; Imada, T.; Shinkai, S. Angew. Chem. Int. Ed. Engl. 1998, 37, 2096 - 2099; Crossley, M. W.; Mackay, L. G.; Tyr, A. C. Chem. Commun. 1995, 1926 - 1927.
(3) Dandliker, P. J.; Diederich, F.; Gisselbrecht, J.-P.; Louati, A.; Gross, M. Angew. Chem. Int. Ed. Engl. 1995, 34, 2725 - 2728; Kimura, M.; Nakada, K.; Yamaguchi, Y.; Hanabusa, K.; Shirai, H.; Kobayashi, N. Chem. Commun. 1997, 1215 � 1216.
(4) Shimizu, Y.; Matsuno, J.; Miya, M.; Nagata, A. Chem. Commun. 1994, 2411 - 2412.
(5) Prathapan, S.; Johnson, T. E.; Lindsey, J. S. J. Am. Chem. Soc. 1993, 115, 7519 - 7520; Wagner, R. W.; Lindsey, J. S. J. Am. Chem. Soc. 1994, 116, 9759 - 9760; Wagner, R. W.; Lindsey, J. S.; Seth, J.; Palaniappan, V.; Bocian, D. F. J. Am. Chem. Soc. 1996, 118, 3996 - 3997; Wagner, R. W.; Seth, J.; Kim, D.; Bocian, D. F.; Holten, D.; Lindsey, J. S. J. Org. Chem. 1998, 63, 5042 - 5049.
(6) The Porphyrin Handbook; Kadish, K. M.; Smith, K. M.; Guilard, R., Eds.; Academic Press: San Diego, 2000.
(7) Wagner, R. W.; Lindsey, J. S.; Turowska-Tyrk, I.; Scheidt, W. R. Tetrahedron 1994, 50, 11097 - 11112.
(8) Collman, J. P.; Zhang, X.; Herrmann, P. C.; Uffelman, E. S.; Boitrel, B.; Straumanis, A.; Brauman, J. I. J. Am. Chem. Soc. 1994, 116, 2681 - 2682; Collman, J. P.; Herrmann, P. C.; Fu, L., Eberspacher, T. A.; Eubanks, M.; Boitrel, B.; Hayoz, P.; Zhang, X.; Brauman, J. I.; Day, V. W. J. Am. Chem. Soc. 1997, 119, 3481 - 3489.
(9) Rose, E.; Kossanyi, A.; Quelquejeu, M.; Soleilhavoup, M.; Duwavran, F.; Bernard, N.; Lecas, A. J. Am. Chem. Soc. 1996, 118, 1567 - 1568.
(10) N. Jux, Organic Letters 2000, 2, 2129 - 2132.
(11) For 2: i. 2,6-bis(methoxymethyl)-4-t-butylbenzaldehyde 1, pyrrole as solvent, BF3*OEt2, RT, isolation of the dipyrromethane; ii. 3 eq. 4-t-butylbenzaldehyde, 2 eq. pyrrole, CH2Cl2 (+1% EtOH), RT; iii. DDQ, CH2Cl2, RT; for 3: i. 2,6-bis(methoxymethyl)-4-t-butylbenzaldehyde 1, pyrrole as solvent, BF3*OEt2, RT, isolation of the dipyrromethane; ii. 1 eq. 4-t-butylbenzaldehyde BF3*OEt2, CH2Cl2 (+1% EtOH), RT, iii. DDQ, CH2Cl2, RT; for 4: 2,6-bis(methoxymethyl)-4-t-butylbenzaldehyde, 1 eq. pyrrole, BF3*OEt2, CH2Cl2 (+1% EtOH), RT, ii. DDQ, CH2Cl2, RT.
(12)
One isomer for mono- and heptakis-substitution, five isomers for bis- and hexakis-substitution (including a pair of enantiomers), five isomers for tris- and pentakis-substitution (including two pairs of enantiomers), and ten isomers for tetrakis-substitution (including three pairs of enantiomers).All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0018 as the message subject of your e-mail.