MICROWAVE SYNTHESIS OF 4-HYDROXYQUINAZOLINES |
 |
[A0071]
Julio A. Seijas*, M. Pilar Vázquez-Tato* , M. Montserrat Martínez
Departamento de Química Orgánica. Facultad de Ciencias de Lugo. Universidad de Santiago de Compostela. Aptdo.
280. 27080-LUGO.
SPAIN
E-mail: [email protected] , [email protected]
Received: 4 August 2000 / Uploaded: 5 August 2000
Recently, we have reported [1]
the microwave enhanced synthesis of 4-aminoquinazolines from anthranilonitrile and
several aromatic nitriles Scheme 1. In the absence of another nitrile, anthranilonitrile
dimerized to gave the corresponding 4-amino-2-(2'-aminophenyl)quinazoline. There we
reported a reduction in reaction times together with an improvement in yields regarding to
the methods using conventional heating.
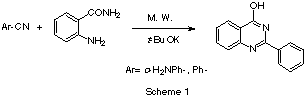 |
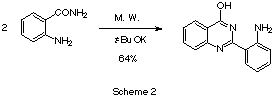
Here we report a similar behavior observed in
anthranilamide, bu in this case instead 4-aminoquinazolines, 4-hydroxyquinazolines were
formed. These 4-hydroxyquinazolines are too compounds with pharmacological interest [2]. We observed that when anthranilamide was heated with 10%
molar ratio of potassium tert-butoxide, we obtained the corresponding
4-hydroxy-2-(2'-aminophenyl)quinazoline Scheme 2 in a 64% yield after two minutes of
heating in a microwave oven. This a yield much higher than the one obtained dimerizing
anthranilamide by the conventional method (17%) [3].
 |
We also heated in a domestic microwave oven a mixture 10:1:10 of anthranilamide:Kt-BuO:benzonitrile
obtaining 4-hydroxy-2-phenylquinazoline Scheme 3 in a 53% yield after three minutes
heating.
|
Although the yields obtained for 4-hydroxyquinazolines are about a 30% lower than those obtained for the analogous 4-amino quinazolines, we think it still constitutes a good alternative for the conventional methods, since yields are good, and reactions are easy to run, its has operational and environmental advantages due to the needless of solvent, and the reactions seems to be easily scaled up.
Acknowledgments: Financial support from DGES (project PB96-0932) is gratefully acknowledged.
1.-Seijas, J. A.; Vázquez-Tato, M. P.; Martínez, M. M., Tetrahedron Lett, 2000, 41, 2215-2217
2.- Hori, M; Iemura, R.; Hara, H.; Ozaki, A.; Sukamoto, T; Ohtaka, H, Chem. Pharm. Bull., 1990, 38, 1286-1291. Jiang, J. b.; Hesson, D.P.; Dusak, B. A.; Dexter, D. L.; Kang, G. J.; Hamel, E. J., J. Med. Chem., 1990, 33, 1721-1728. Hamel, E.; Lin, C. M.; Plowman, J.; Wandg, H. K.; Lee, K. H.; Paull, K. D., Biochem. Pharmacol., 1996, 51, 53-53.
3.- Pakrashi, S. C., J. Org. Chem., 1971, 36, 642-645.
 |
All comments on this poster should be sent by e-mail to (mailto:[email protected]) [email protected] with A0071 as the message subject of your e-mail. |  |