Microwave Activation in the Chemistry of Hantzsch 1,4-Dihydropyridines. An Overview
Jean Jacques Vanden Eynde and Annie Mayence
University of Mons-Hainaut
Organic Chemistry Department, Place du Parc
20, B – 7000 Mons (Belgium)
[email protected]
Dedicated to Professor E. Anders (Friedrich
Schiller University, Jena, Germany) on the occasion of his 60th
birthday
Received: 3 September 2001 / Uploaded 4 September 2001
INTRODUCTION
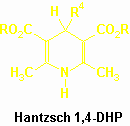
Hantzsch 1,4-dihydropyridines (1,4-DHP : dialkyl 1,4-dihydro-2,6-dimethylpyridine-3,5-dicarboxylates) remain the most important class of calcium channel modulators (F. Bossert et al., Angew. Chem. Intern. Ed. Engl., 20, 762, 1981 ; H. Nakayama et al., Heterocycles, 42, 901 1996). Some of them, such as Nifedipine, Amlodipine, Isradipine, …, have been commercialized and are known as vital drugs in the treatment of angina and hypertension, due to their vasodilator properties.

The success of those calcium antagonists has led to the development of novel synthetic strategies to improve their classical methods of preparation (Gordeev et al., J. Org. Chem., 61, 924, 1996 ; J.G. Breitenbucher et al., Tetrahedron Lett., 41, 4311, 2000). Microwave activation stands among the alternative routes proposed the past decade.
Aromatization of 1,4-DHP has also attracted considerable attention in recent years, essentially because it has been established that the metabolism of those drugs involves an oxidation step that is catalyzed, in the liver, by cytochrome P-450 (Böcker et al., J. Med. Chem., 29, 1596, 1986). Consequently, that reaction has been the subject of a large number of studies and a plethora of reagents has been utilized to provide high-yielding methods for the preparation of the corresponding Hantzsch pyridines. In that field, surprising results have been collected when the reactions are performed under microwave irradiation.
Hantzsch 1,4-DHP Synthesis
The first 1,4-DHP were obtained more than one century ago by Hantzsch (Justus Liebigs Ann. Chem., 215, 1, 1882). The proposed experimental method remains the most widely used protocol to access to those heterocyclic derivatives. A mixture of an aldehyde, a $ -ketoester and ammoniac in ethanol is heated under reflux for several hours. That multicomponent reaction often affords the target compounds in good yields. Modified procedures involve the use of preformed Knoevenagel adducts between the aldehyde and the ketoester or the use of preformed enaminoesters (represented in the E form to clarify the scheme).
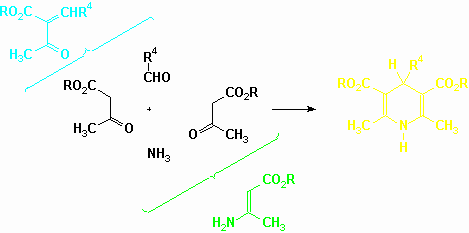
The pioneering report on the use of microwaves to promote Hantzsch 1,4-DHP synthesis is signed by Alajarin (Synlett, 297, 1992). The group prepared a series of 4-aryl derivatives in a domestic oven by the classical multicomponent method (aldehyde : 15 mmol ; alkyl acetoacetate : 43 mmol ; ammoniac : 30 mmol ; ethanol : 3 ml). Yields range from 15 to 52 % for a reaction time of 4 minutes. Literature procedures for the formation of the same compounds requires a reflux period of 12 hours and yields range from 36 to 50 %. In 1995 the same group extended its work (Synthesis, 389, 1995) to the synthesis, in a domestic aven, of 3,5-unsymmetrically substituted 1,4-DHP starting from arylmethyleneacetoacetate (8 mmol) and 3-aminocrotonate (4 mmol) in ethanol ((4.5 ml). Just like for the preparation of the symmetrical compounds, significant reaction time improvements have been observed, The same year Zhang (Synth. Commun., 25, 857 1995) described the preparation of four 4-aryl 1,4-DHP from 3-aminocrotonate (20 mmol), methyl acetoacetate (20 mmol) and arylaldehydes (20 mmol) in the absence of solvent in a domestic oven. Yields from 59 to 77 % are reported and optimized heating period does not exceed 10 minutes. The same building blocks have been used by Khadilkar (Ind. J. Chem., 34B, 652 1995) but he conducted the reactions in ethanol (volume not reported ; 3-aminocrotonate : 10 mmol ; methyl acetoacetate : 14 mmol ; arylaldehyde : 10 mmol). The experiments were performed in a domestic oven for 3 to 5 minutes. Interestingly, Khadilkar (Tetrahedron Lett., 39, 1117, 1998) has also described the formation, in a domestic oven, of 1,4-DHP in an aqueous hydrotrope solution (50% butylmonoglycolsulphate : 5 ml) from 3-aminocrotonate (10 mmol) methyl acetoacetate (14 mmol) and aliphatic or aromatic aldehydes (10 mmol). Finally, the coupling of microwave heating (in a domestic oven) with the use of a mineral solid support (alumina : 2 g) has been exploited (M. Suarez et al., Heterocycl. Commun., 2, 275, 1996) to obtain an unsymmetrical 1,4-DHP from methyl 3-aminocrotonate (3 mmol), ethyl acetoacetate (3 mmol) and benzaldehyde (3 mmol). A catalytic amount of DMF (0.5 ml), as an energy transfer medium to attain higher temperatures, was added to the reaction mixture.
More recently, we described (J.J. Vanden Eynde, Tetrahedron, 55, 2687 1999) soluble polymer-anchored 1,4-DHP. Such polymers as well as the parent 4-unsubstituted 1,4-DHP can now be synthesized under solvent-free conditions (D. Barbry, results submitted for publication). The original experimental procedure yielding that latter compound has been carried out in a monomode oven (Synthewave 402 from Prolabo) and requires 100 seconds only. The strategy involves ethyl acetoacetate (20 mmol) and hexamethylenetetramine 8 mmol) as the source of the ammonia-formaldehyde mixture. Ammonium acetate (10 mmol) was added to the reaction medium in order to obtain the stoichiometric balance between ammonia and methanal.
Aromatization of Hantzsch 1,4-DHP
The numerous reports on aromatization of Hantzsch 1,4-DHP (excluding the papers dealing with microwave-mediated experiments) can be summarized as follows :
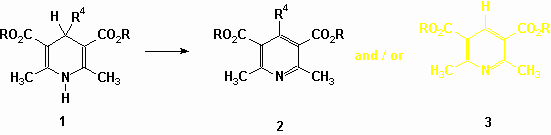
The first microwave-mediated aromatization of 1,4-DHP was reported in 1991 by Alvarez (Synth. Commun., 21, 619 1991 ; ibidem, 21, 2137, 1991). The authors observed that several 1,4-DHP (0.5 g) could readily be oxidized, in a domestic oven, by a mixture (5.0 g) of MnO2 on a Mexican bentonite clay in the absence of solvent. Short reaction times (10 minutes) and excellent yields characterize the procedure. However, the most striking results deal with the obtaining of mixtures of 4-alkylpyridines (2) and the 4-unsubstituted pyridine (3) when starting from 1,4-DHP bearing a methyl, ethyl or propyl group in position 4. In contrast, two years later the group related (O. Garcia et al., Tetrahedron Lett., 34, 623, 1993) that the same 4-alkyl 1,4-DHP (0.25 g) do not undergo the dealkylation process when they are irradiated for 1 minute in the presence of a HNO3/Mexican bentonite clay system (2.5 g) in a domestic oven. Varma (J. Chem. Soc. Perkin Trans. 1, 1755, 1999) also studied that aromatization reaction. He demonstrates that solid state oxidation of 1, 4-DHPs (1mmol) using elemental sulfur (1,3 mmol) and microwave activation in a domestic oven yields the dehydro derivatives, whichever the 4-substituent is.
Our experience in the field of 1,4-DHP chemistry (Experimental Oncology, 22 suppl. 2, 16, 2000 ; Intern. J. Med. Biol. Environm., 18, 89, 2000) led us to initiate a study of the solvent-free aromatization of Hantzsch 1,4-DHP upon classical and microwave heating in the absence of external oxidant except air. Firstly we established the profile of the increase of temperature of a bath of alumina under given power settings in a domestic microwave oven and we managed to maintain a bath of alumina at the same final temperature (200 °C) on a hot plate. Then, taking care to replace the alumina after each experiment, we put a round-bottom flask filled with 10 mmol of the 1,4-DHP in the bath and submitted it either to microwave irradiation or to classical heating for the same given period (10 min). We observed that the 4-phenyl derivative is stable under both experimental conditions. The 4-isopropyl derivative yields the 4-unsubstituted pyridine (3) in both experimental conditions (around 50 % yield) and the 4-propyl derivative yields a mixture of the 4-alkylated pyridine (2) and the 4-unsubstituted pyridine (3) under both experimental conditions : the overall yield is highly dependent to slight modifications of the temperature of the bath (from 25 % to 80 % for a change of temperature in a range of less than 20 °C) but the ratio remains rather constant and in favor of the dealkylated product (2 : 3 = 1 : 4). Let us mention that the 4-propylpyridine (2) is stable upon heating and therefore the formation of the 4-unsubstituted heterocycle (3) cannot be explained by a dealkylation of 2. Those preliminary results clearly indicate that specific microwave effects do not seem to play a important role in the experiments. On the other hand our observations reveal a particular behavior of Hantzsch 1,4-DHP upon heating and a dramatic influence of the temperature on their susceptibility to be oxidized by air (oxygen) above their melting point. Therefore when considering the solvent-free aromatization of 1,4-DHP, the rate of the oxidation process induced by air should not be neglected. As that rate is temperature-dependent, the presence of an inorganic solid in the reaction media emerge as an additional factor that can modify the yields due to the thermal conductivity properties of that inorganic substance, especially under microwave irradiation. Further investigations are currently in progress in our laboratory.
Less ambiguously let us finally report the use, by Barbry (results submitted for publication), of the parent 1,4-DHP (6 mmol) in the reduction of activated carbon-carbon double bonds (4 mmol) on silica gel (4 g) under microwave irradiation without solvent in a domestic multimode oven (8 min) and in the Synthewave monomode oven (4-6 min).
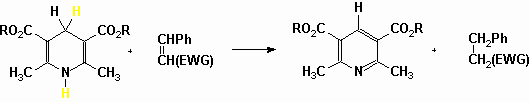
Domino Synthesis of Hantzsch Pyridines
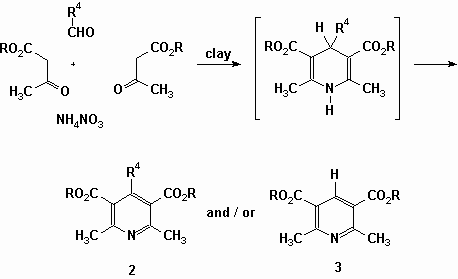
Mention should be made that there are two papers describing the domino synthesis of Hantzsch pyridines. Both groups start from ?-ketoesters, aldehydes and ammonium nitrate, as a source of ammonia and oxidizing species, in the presence of a bentonite clay. The reactions were performed in domestic ovens. The published results are intriguing as they are somehow contradictory. In the absence of solvent, Penieres (Heterocycl. Commun., 2, 359, 1996) isolated from isobutyraldehyde (20 mmol; 40 mmol of the ketoester and 20 mmol of NH4NO3 on 5 g of clay) the alkylated pyridine (2) as the major product. On the other hand he obtained the 4-unsubstituted pyridine (3), in major yield, when n-butyraldehyde or benzaldehyde are used. That later observation also contrasts with the claims of Cotterill (Tetrahedron Lett., 39, 1117, 1998) who carried out the syntheses in DMF (35 ?l) from diverse arylaldehydes (0.1 mmol; 2 eq. of ketoester; 100 mg of a 5:1 w/w mixture of bentonite and NH4NO3)in a combinatorial approach and do not mention the presence of that parent pyridine 3 in the final mixtures.
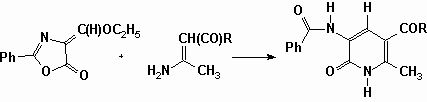
In addition to those two papers, let us compile the domino preparation of pyridinones structurally related to Hantzsch pyridines under microwave irradiation in a domestic oven. (Vanden Eynde et al., Synth. Commun., 27, 3683, 1997).
High-Throughput Synthesis
Whereas Cotterill (Tetrahedron Lett.,39, 1117, 1998) reported the domino synthesis of Hantzsch pyridines in the 96-well microtiter plate format in a domestic microwave oven (vide supra), a recent publication ( Ohberg et al., Synlett, 1296, 2001) claims the use of a professional oven for the synthesis of 24 1,4-DHP from 6 different aldehydes and 4 dicarbonyl derivatives in aqueous ammonia at 140 - 150 °C in sealed vessels. The desired products were obtained within 10 – 15 minutes in yields ranging from 39 to 89 %.
Conclusion
Hantzsch 1,4-DHP and their first metabolites,
because of their pharmacological profiles, still attract much attention as
demonstrated by the development of novel synthetic protocols enabling the access
to those heterocyclic derivatives in excellent yields and short reaction times.
In recent years microwave irradiation has been successfully applied to promote
Hantzsch 1,4-DHP and their behavior towards oxidants. Whereas the construction
of 1,4-DHP has been performed in solution or in dry media, protocols yielding
the pyridine derivatives involve solvent-free conditions only. In those cases
unexpected results have been reported thus initiating and requiring further
debates on the subject.
(No reference)