[E001]
 |
Determination of Chain Branching in Solid Epoxy
Resins Using 1H NMR Spectrometry Jarosław Górczyk, Dariusz BogdałDepartment of Polymer Chemistry and Technology, |
| * e-mail: [email protected] | |
[E001]
 |
Determination of Chain Branching in Solid Epoxy
Resins Using 1H NMR Spectrometry Jarosław Górczyk, Dariusz BogdałDepartment of Polymer Chemistry and Technology, |
| * e-mail: [email protected] | |
Abstract: Solid epoxy resins were synthesized both under conventional heating and microwave irradiation (reactions were performed in a Multi-mode microwave reactor “Plazmatronika”, microwave frequency-2,45GHz, maximum of microwave power-300W). The H1 NMR spectra were made before and after the reaction of solid epoxy resin with Trichloroacetyl isocyanate. It allowed us to determine the degree of branching.
Keywords: H1 NMR spectrometry, solid epoxy resin, microwave irradiation, polyaddition
Epoxy resins are produced from the begining of 50-ths. Because of their unique properities, e.g. excellent chemical and electrical resistance, very good adhesion to different kinds of materials and good heat resistance, they are willingly used in various kinds of everyday life1). Solid epoxy resins (Epoxy Value EV=0,25-0,02; Mn=1000-10000), (which are used in industry of varnishes and dyes), are mostly synthesised in according to indirect method2,3,4). This method, called "advancement" process is based on polyaddition of Bisphenol A to Low-molecular-weight epoxy resin (Epoxy Value EV=0,58-0,35; Mn=370-500) or Middle-molecular-weight epoxy resin (Epoxy Value EV=0,30-0,15; Mn=500-1000) in the presence of a catalyst5,6). It is possible to synthesize such resins in a batch or continous process, under conventional heating or microwave irradiation.
Schematic reaction of synthesis solid epoxy resins is shown in Figure 1.
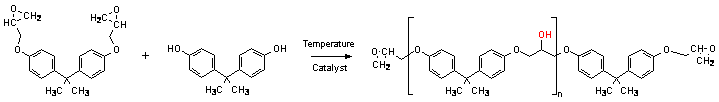
Figure 1. "Advancement" process (polyaddition of Bisphenol A to Low-molecular-weight epoxy resin).
As we can see in a Figure 1 each molecule
contains as many aliphatic hydroxyl groups as there
are repeat units in the linear structure. These hydroxyls are a potential
brenching points. So that for every branch point in the nonlinear structure
there is one hydroxyl-carbinol methine pair less in comparison with the linear
structure of equal molecular weight. The H1 NMR method of determining
branching points relies on involving reaction between hydroxyl groups presented
in the solid epoxide resin and Trichloroacetyl isocyanate7,8,9,10).
The urethane group is formed as a result of that reaction. For each sample the
H1 NMR spectra are made two times before and after the reaction.
Hypothetical H1 NMR spectra before (Figure 2) and
after (Figure 3) the reaction of epoxy resin with
Trichloroacetyl isocyanate are shown below 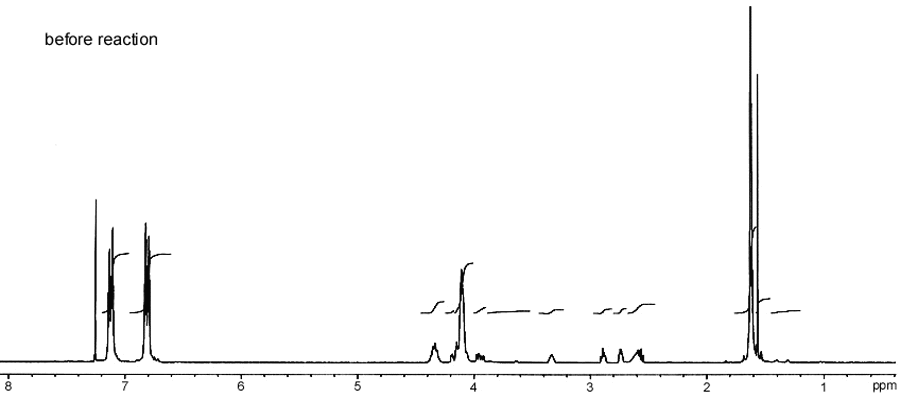 Figure 2. H1 NMR spectrum before the
reaction of solid epoxy resin with Trichloroacetyl isocyanate.
Figure 2. H1 NMR spectrum before the
reaction of solid epoxy resin with Trichloroacetyl isocyanate. 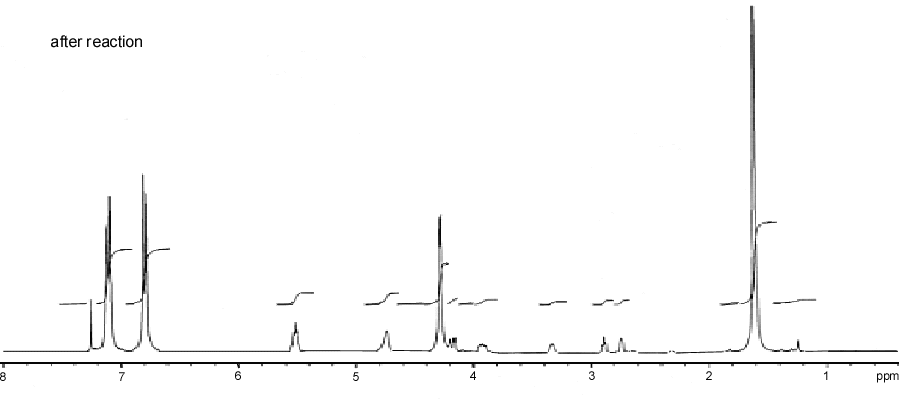 Figure 3. H1 NMR spectrum
after the reaction of solid epoxy resin with Trichloroacetyl isocyanate.
Figure 3. H1 NMR spectrum
after the reaction of solid epoxy resin with Trichloroacetyl isocyanate.
The total number of methine protons Zmethine can
be describe as a fifth part of sum the reduced integration of methine and
methylene signals before reaction:
Zmethine = 1/5 · (Z4.1 - Z4.3)
The number of methine protons in a branched molecules Zbranched however can be describe as a difference between Zmethine and the reduced integration of methine signal after reaction with Trichloroacetyl isocyanate:
Zbranched = Zmethine - Z5.5
where:
Z4.1-reduced integration of methylene signal before
reaction
Z4.3-reduced integration of methine signal before
reaction
Z5.5-reduced integration of methine signal after
reaction
So that the degree of branching f can be calculated
according to the formula:
f = (Z4.1 - Z4.3 - 5Z5.5) / (Z4.1 - Z4.3)
It is also easy to estimate the number of branche points per moleculer r according to the formula:
r = f · (Mn - 340.4) / 284.4
Persentage of chain branching can be expressed as a ratio of the number of branche points per moleculer r to the number of repeat units n. Where:
n = 2 · (EEW - 170.2) / 284.4
All appropriate data are stored in Table
2.
The general procedure for the syntheses of solid epoxy resins
can be described as follows:
Appropriate amount of Bisphenol A was added
to Low-molecular-weight epoxy resin (Epoxy Value EV=0.57 mol/100 g) to obtain
required increasing the molecular weight during reaction to the desired level.
The calculated molar rate of Bisphenol A to Low-molecular-weight epoxy resin was
3 : 4. The mixture was stirred at 160°C, in a
Multi-mode microwave reactor “Plazmatronika” - Figure 2 (microwave frequency -
2,45GHz, maximum of microwave power - 300W), for time necessary to obtain Epoxy
Value about 0,11. 100W of microwave power was used. Every 5 minutes a small
sample of epoxy resin was taken from the mixture to determine the Epoxy
Value. After the reaction epoxy resin was cooled down and powdered.

|
Plazmatronika microwave reactor implements novell Concentrated Microwave Field (CMF) which provides the microwave field focused into the reaction vessel. The temperature can be measured at the bottom of the reaction chamber using Infra-red thermometry with fine beam focusing (http://www.plazmatronika.pl). |
Figure 4. A Multi-mode microwave reactor "Plazmatronika". | |
Epoxy Values of synthesised resins were determined according to the PN-87/C-89085/13. All GPC analyses were obtained on a GPC chromatograph (“Knauer”). System of three columns was used: 2×PL-gel (300×7.5 mm; dimension of grains 3mm and type of pore Mixed-E) with one precolumn. The temperature of measurement was 30°C and THF was a solvent. Results of all analyses are presented in Table 1.
The general
procedure for determination the degree of branching of solid epoxy resins can be
described as follows:
Aproximately 10 mg of solid epoxy resin was
dissolved in 0.5 ml of Deuterochloroform. The solution was scanned on a
Merkury-300 "Varian" spectrometer and the peaks were integrated. Then 10 mg
of Trichloroacetyl isocyanate was added and after 5 minutes a few drops of
Deuterium oxide. The solution was scanned second time and the peaks were
integrated. Results of all analyses are presented in Table 2.
Table 1. Results of determination of molecular weight distribution of solid epoxy resins by GPC chromatography.
| Epoxy resin sample |
Reaction heating |
Reaction time [min] |
Catalyst |
Epoxy Value [mol/100g] |
GPC analysis | ||
| Mn | Mw | Pd | |||||
| A160A | microwave |
65 |
0.5 |
0.110 |
2140 |
3780 |
1.77 |
| A160B |
40 |
1 |
0.113 |
2150 |
3930 |
1.83 | |
| A160C |
20 |
5 |
0.104 |
2470 |
3390 |
1.83 | |
| K160A | conventional |
120 |
0.5 |
0.106 |
1790 |
3130 |
1.75 |
| K160B |
80 |
1 |
0.111 |
2180 |
4000 |
1.84 | |
| K160C |
35 |
5 |
0.100 |
2380 |
5010 |
2.10 | |
GPC analyses shows that all synthesized solid epoxy resins have comparable molecular weight and polydispersity.
Table2. Results of determination of chain branching in solid epoxy resins by H1 NMR spectrometry.
| Epoxy resin sample |
Z4.1 | Z4.3 | Z5.5 | EEW | Number of branche points per moleculer r |
Degree
of branching f |
Number of repeat units n |
Persentage of chain branching [%] |
| A160A | 3.455 | 0.805 | 0.774 | 909 | 0.48 | 0.09 | 5.2 | 9.24 |
| A160B | 3.433 | 0.782 | 0.782 | 885 | 0.36 | 0.07 | 5.0 | 7.16 |
| A160C | 4.134 | 0.792 | 0.775 | 962 | 1.19 | 0.21 | 5.6 | 21.38 |
| K160A | 3.397 | 0.787 | 0.770 | 943 | 0.44 | 0.08 | 5.4 | 8.09 |
| K160B | 3.516 | 0.814 | 0.788 | 901 | 0.47 | 0.09 | 5.1 | 9.15 |
| K160C | 3.587 | 0.797 | 0.749 | 1000 | 0.85 | 0.15 | 5.8 | 14.57 |
The degree of branching is in the range from 0.36 to 1.19. It is consistent with the literature data. As one can see it is better not to use too much catalyst. The catalyst content 5·10-3[mol] has already caused on a large scale ariseing the chain branching. The optimal reaction conditions for processes provided under microwave irradiation were found to be: 40 minutes, temperature 160°C, content of catalyst 1·10-3[mol] and 100W of microwave power. Shortening of the reaction time for all reactions provided in microwave reactor in comparison to conventional heating were observed. Important is that all solid epoxy resins synthesized both under microwave irradiation and conventional heating have comparable physico-chemical properties.
3)
Z. Brojer, Polimery, 25, 205 (1980).4)
B.Szczepaniak, J. Rejdych, Polimery, 27, 236 (1982). Pat. USA 6,022,931 (2000).6)
Pat. USA 6,262,189 B1 (2001). H.D. Mak, M.D. Rogers, Analytical Chemistry, 44, 837 (1972).8)
M.D. Rogers, Journal of Applied Polymer Science, 16, 1953 (1972).9)
H. Batyer, Zahir S.A.: Journal of Applied Polymer Science, 19, 585 (1975).10)
H. Batyer, Zahir S.A.: Journal of Applied Polymer Science, 19, 601 (1975).