Entropy 2000, 2[3], 106-141
Inquiries into the Nature of Free Energy and Entropy in Respect to Biochemical Thermodynamics
Clinton D. Stoner
Department of Surgery, Ohio State University, Columbus, Ohio 43210, U.S.A.
E-mail: [email protected]
Received: 2 May 2000 / Accepted: 29 July 2000 / Published: 9 August 2000
Abstract:
Free energy and entropy are examined in detail from the standpoint of classical
thermodynamics. The approach is logically based on the fact that thermodynamic
work is mediated by thermal energy through the tendency for nonthermal energy
to convert spontaneously into thermal energy and for thermal energy to distribute
spontaneously and uniformly within the accessible space. The fact that free
energy is a Second-Law, expendable energy that makes it possible for thermodynamic
work to be done at finite rates is emphasized. Entropy, as originally defined,
is pointed out to be the capacity factor for thermal energy that is hidden with
respect to temperature; it serves to evaluate the practical quality of thermal
energy and to account for changes in the amounts of latent thermal energies
in systems maintained at constant temperature. With entropy thus operationally
defined, it is possible to see that TDS°
of the Gibbs standard free energy relation DG° = DH° - TDS°
serves to account for differences or changes in nonthermal energies that do
not contribute to DG° and that, since
DH° serves to account for differences
or changes in total energy, complete enthalpy-entropy (DH°-TDS°)
compensation must invariably occur in isothermal processes for which TDS°
is finite. A major objective was to clarify the means by which free energy is transferred
and conserved in sequences of biological reactions coupled by freely diffusible
intermediates. In achieving this objective it was found necessary to distinguish
between a 'characteristic free energy' possessed by all First-Law energies in
amounts equivalent to the amounts of the energies themselves and a 'free energy
of concentration' that is intrinsically mechanical and relatively elusive in
that it can appear to be free of First-Law energy. The findings in this regard
serve to clarify the fact that the transfer of chemical potential energy from
one repository to another along sequences of biological reactions of the above
sort occurs through transfer of the First-Law energy as thermal energy and transfer
of the Second-Law energy as free energy of concentration.
Keywords:
Temperature; Latent thermal energy; Chemical potential energy;
Enthalpy-entropy compensation; Hydrophobic effect; van't Hoff enthalpy; Free
energy transfer and conservation.
Full text in PDF form, 223 K
Introduction
Despite the assurance of the First Law of Thermodynamics
that energy is invariably conserved, we often concern ourselves with the conservation
of energy. This apparent inconsistency
obviously must arise from the fact that the ambient thermal energy into which
high-grade thermal energy and most other forms of energy tend to degrade has
relatively little 'available work potential'. And it must be this so-called
free energy rather than an actual energy that we ordinarily seek to conserve,
'actual energy' referring to any of the various energies recognized by the First
Law.
The primary objectives in pursuing this study
were to achieve fully operational understandings of free energy and its close
relative entropy and thereby to clarify apparent inconsistencies of the above
sort and to deal with a number of other problems that tend to make the laws
and fundamental equations of thermodynamics generally difficult to interpret
and understand. Some of the problems and their persistence appear to derive
from the common practice of viewing entropy as a measure of disorder. Entropy
as disorder is not measurable as such and thus is not operational. Whatever
the case, the findings here suggest that several of the difficulties that commonly
plague the interpretation of thermodynamic phenomena can be resolved by identifying
and distinguishing between the various energies involved and taking into consideration
the practical qualities of the actual energies in respect to free energy.
The original objective was simply to clarify
the means by which free energy is transferred and conserved in sequences of
stationary-state biological reactions coupled by freely diffusible intermediates.
The reported findings in this regard serve to emphasize the need to acknowledge
the existence of an intrinsically mechanical, phantom-like free energy that
can disappear without a trace and thereby appear to be free of actual energy.
Energy, Temperature, Work, Work Potential, and Free Energy
Energy is usually defined in terms of a capacity
of something to do work. Actual energy of matter may be classed broadly into
two interconvertible varieties: energy of motion (kinetic energy) and energy
of constrained motion (potential energy). Energy of motion can be subdivided
into directed and undirected varieties. Disregarding the radiant form, thermal
energy is an actual energy of undirected (random) motion of the individually
mobile particulate constituents of macroscopic amounts of matter and is a mechanical
kind of energy into which all other forms of actual energy tend to convert.
Temperature is a property of macroscopic amounts of matter and serves to gauge
the intensity of the thermal energy. Thermal energy transfer occurs spontaneously
and net transfer along a gradient of temperature is a one-way process, occurring
only from higher to lower temperature. In consequence, macroscopic amounts of
matter in thermal contact with one another tend to be at the same temperature,
a fact of sufficient fundamental importance to merit belated designation as
the Zeroth Law of Thermodynamics.
Work may be defined roughly as any activity
that is energetically equivalent to lifting a weight. Since it exists only at
the time it is being performed, work is generally viewed both as a nonthermal
actual energy in transit between one form or repository and another and as a
means of nonthermal actual energy transfer. We shall be concerned here only
with 'thermodynamic work' (i.e., only with work in which the transit
of nonthermal actual energy between forms or repositories occurs through intermediary
thermal energy). Accordingly, 'conservative work', such as that done in direct
conversions between gravitational potential energy and directed motional energy
of matter, shall be ignored. Transfers of actual energy in thermodynamic work
processes require specific mechanisms that depend on the nature of the energy.
Thermal energy transfer occurs by conduction, convection, and radiation.
Consistent with the reciprocity in the definitions
of work and energy, work potential shall be considered here to be the total
potential of an energy for doing work. Although in principle all actual energies
are equivalent to their work potentials and thus are equivalent with respect
to work potential, they differ with respect to available work potential and
thus differ with respect to quality, the quality being higher the greater the
availability of the work potential. The availability of the work potential of
an actual energy depends on the nature of the energy and on the conditions under
which work is done. Whereas the work potential of thermal energy is completely
unavailable at constant temperature, that of all other forms of actual energy
can in principle become available at constant temperature as a result of (i)
the tendency of nonthermal actual energy to convert spontaneously into thermal
energy of quality (temperature) exceeding that of the ambient thermal energy
and of (ii) the capacity of relatively high-quality thermal energy to
do mechanical work through its tendency to distribute spontaneously and uniformly
within the accessible ambient space. In the course of any work thus done, actual
energy and work potential are invariably conserved, whereas energy quality,
or available work potential (free energy), is invariably consumed. As will be
emphasized below, the amount of free energy consumed in the course of a thermodynamic
work process depends greatly on the magnitude of the differential in quality
between the intermediary and ambient thermal energies and thus on how fast the
work is done.
Since it is possible in principle for thermodynamic
work to be done under conditions of the rate and the differential in thermal
energy quality being extremely small, any nonthermal actual energy can be considered
to possess in principle an amount of free energy equivalent to the amount of
the energy itself. In consequence, all actual energies other than thermal energy
may be viewed as being completely interconvertible through mechanical work at
ordinary ambient temperatures and thus may be viewed equally as energy of high
quality. However, owing to differences in barriers to conversion, to differences
in complexity of required mechanisms, and to consequent unavoidable differences
in losses of free energy in real conversion processes, the qualities of the
various nonthermal actual energies differ from the standpoint of practical processes.
Because gravitational potential energy can be converted directly and unimpededly
into mechanical work, the energy conserved in the course of lifting a weight
is generally viewed as the form of nonthermal actual energy having the highest
quality.
Although completely unavailable at constant temperature, the work potential
of thermal energy can be realized at the expense of a decrease in the temperature
of the energy and thus for thermal energy having a particular temperature the
work potential may be viewed as being potentially available. However, because
complete conversion of a given amount of thermal energy into a nonthermal actual
energy would require a decrease in the temperature of the given amount to absolute
zero, the potential availability of the work potential of thermal energy is
quite limited and that of ambient thermal energy at ordinary ambient temperatures
is practically nil.
Thermodynamics of Gases
The fundamental aspects of thermodynamics
are based largely on energy changes associated with changes in the state properties
pressure, volume, and temperature of an ideal gas in accordance with the ideal
gas equation: PV = nRT. In consequence, the nature of
free energy and its relationship to entropy are best seen by examining the predictions
of the laws of thermodynamics in respect to compression and expansion of a gas
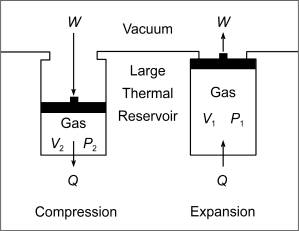
of this kind. Such will be done here by considering an ideal gas confined within
a rigid cylinder of the sort shown in Figure 1. The walls of the cylinder are
assumed to be permeable to thermal energy and in contact with a thermal reservoir
that is sufficiently large and conductive to validate the assumption that its
temperature remains constant despite transmissions of thermal energy between
it and the gas. The gas is assumed to be confined to the cylinder and separated
from a vacuum by a massless and frictionless piston by which it can be compressed
and allowed to expand.
|
|
Figure 1.
Compression and expansion of an ideal gas in a rigid-cylinder, piston
system. The piston is assumed
to be massless and frictionless and the temperature of the thermal reservoir
is assumed to be constant. W = an amount
of mechanical work; Q = an amount of thermal
energy; V = volume of the gas; P = pressure
of the gas.
|
It is important to note that, since ideal
gases and solutes in ideal solution are virtually identical in respect to mechanical
thermodynamic properties at normal ambient temperatures and pressures [1], the Figure 1 system is also relevant to the thermodynamics
of dissolved substances. Thus, although the kinetics would be much different,
the same thermodynamic relationships would apply if (i) the gas of Figure
1 were a solute in ideal solution, (ii) the vacuum were replaced by the
pure solvent, and (iii) the piston were selectively permeable to the
solvent. If such were the case, the concentration and osmotic pressure of the
gas as a solute would correspond to the concentration and pressure of the gas
as a gas, and the thermodynamic relationships would be largely independent of
the pressure on the solvent within the normal atmospheric range of pressure.
According to the ideal gas equation and the First Law of Thermodynamics, if
an amount of work W were done on an ideal gas in compressing it from
the maximum volume V1 to a volume V2,
an amount of thermal energy Q equivalent to the work done would be transmitted
from the gas to the reservoir in the course of the gas achieving thermal equilibrium
with its surroundings during and after the compression. According to the ideal
gas equation, if the compression of one mol of the gas were conducted reversibly
(i.e., sufficiently slowly that the gas would remain in virtual thermal
equilibrium with the reservoir), a minimum amount of work Wrev
energetically equivalent to RT ln(V1 /V2)
of gravitational potential energy would be required to compress the gas, and,
according to the First Law, an equivalent amount of thermal energy Qrev
would be transmitted from the gas to the reservoir during the compression. In
the course of this conversion of energy of the highest quality into the ambient
thermal energy, the gas would be endowed with a potential for conversion of
ambient thermal energy into an amount of work equivalent to that done in compressing
it, as is obvious from the specifications that the temperature of the reservoir
be constant and that the compression be conducted reversibly. In other words,
if one mol of the gas were compressed isothermally (reversibly) from V1
to V2, an amount RT ln(V1 /V2)
of actual energy equivalent in quality to gravitational potential energy would
be converted into an amount RT ln(V1 /V2)
of ambient thermal energy plus an amount RT ln(V1 /V2)
of an energy of the gas which is ordinarily ignored when encountered in this
context, but which obviously must be viewed as being free energy. If we were
to consider this free energy to be actual energy, the First Law of Thermodynamics
would of course appear to be very seriously in error. That it is not in error
is evident from the fact that, if the free energy were not used for reversing
the degradation of the nonthermal actual energy into ambient thermal energy,
it would simply disappear, never to reappear. Thus, if the compressed gas were
allowed to expand from V2 to V1 without doing work on the surroundings, as would be the case if the massless
and frictionless piston holding the gas in the compressed state (Figure
1) were suddenly released, it would do so rapidly without a net change in
actual energy of any kind in either the gas or its surroundings [2].
If free energy is not actually energy, what,
then, is it? Since in the course of the compression phase of the above cyclic
process it would become dissociated from the actual energies W and Q,
it appears in this case to be an available work potential that is free of actual
energy. The facts (i) that complete recovery of the reversibly imparted
free energy could be achieved only if the compressed gas were allowed to expand
and do work at an infinitesimal rate and (ii) that, if the gas were allowed
to expand without doing work, it would do so at the maximum possible rate with
complete loss of the free energy suggest that free energy expenditure is what
makes things happen within finite amounts of time. Accordingly, by combining
the well-established equations of steady-state kinetics with those of classical
equilibrium thermodynamics, it is possible to show quantitatively for stationary-state
(apparent-equilibrium) reactions that the faster the reaction, the greater the
amount of free energy consumed [3].
To see clearly the nature of the above free
energy, we must consider the properties of an ideal gas in respect to actual
energy. Gases in molar amounts at ordinary temperatures generally consist of
very large numbers of very small particles that are moving randomly at very
high speeds. They tend to occupy uniformly the entire space accessible to them
and through collisions of the particles exert pressure on whatever confines
them, thereby tending to increase the accessible space. The particles of an
ideal gas are assumed to collide with perfect elasticity and to have volumes
that are negligibly small in relation to the space available for their translation.
In addition, the particles are assumed (i) to be devoid of attractive
and repulsive forces, (ii) to be free of influence by fields of force
such as the gravitational field, and (iii) to possess per mol a total
amount of actual energy E which depends only on the temperature. According
to these assumptions, the actual energy of an ideal gas consists exclusively
of thermal energy and differences in this energy on a per mol basis between
states at the same temperature are zero. Thus, in the above considerations,
DE for the gas was assumed to be zero.
We may now inquire as to the nature and source
of the thermal energy that would appear in an ideal gas as it is compressed.
The thermal energy must come from kinetic energy transmitted by the piston to
the gas particles as the piston is moved toward the particles, this despite
the fact that an amount of thermal energy Qrev would be imparted
even if the piston were moved extremely (infinitely) slowly. The faster the
piston is moved the greater the amount of energy required and thus the greater
W and Q must be. This implies that the temperature would be determined
exclusively by the translational kinetic energies of the particles and that
in the course of thermal equilibration with the reservoir, the kinetic energies
on average would return to their initial values, leaving only a diminished volume
and a consequent augmentation of the pressure to account for the deposition
of free energy in the gas. Accordingly, experimental observations on real gases
suggest that allowing an ideal gas to expand without doing work (i.e.,
without the particles expending kinetic energy) would not affect its temperature
[2].
According to the ideal gas equation, PV would not change in the course
of an isothermal change in the state of such a gas on a per mol basis. This
being the case, RT ln(V1 /V2) for an isothermal
compression would be equivalent to RT ln(P2 /P1) and thus for such a compression one
could attribute the consequent increase in free energy either to the decrease
in the volume or to the increase in the pressure. That it would be appropriate
to attribute the increase only to the increase in pressure is evident from the
facts (i) that driving forces for change are determined only by intensive
variables [4] and (ii) that pressure is the only
intensive variable of the system under consideration.
It may be noted that, since there would be
no difference in actual energy between the states, the increase in free energy
can also be regarded as being due to an increase in the density of the translational
kinetic energy or in the degree to which the translational motions of the gas
particles are constrained, in which cases one can readily see that the free
energy would possess an attribute of potential energy. In addition, since for
an isothermal change the pressure and concentration would vary in direct proportion
to one another, the free energy can also be regarded as being an energy of concentration.
In fact doing so is preferable for the purposes of this study because differences
in this kind of free energy between the reactants and products of biochemical
reactions are ordinarily determined according to differences in concentrations.
As will be pointed out below, the nature of this 'free energy of concentration'
is fundamentally the same for a system in which only chemical work is possible
as for the system here (Figure 1) in which only mechanical work is assumed possible.
Free Energy in Relation to Entropy
Since free energy is not actually energy from the standpoint of the First Law
and can disappear without a trace when of the concentration variety, it is necessary
to account for its consumption in terms of the actual energies. As indicated
above, the actual energies W and Q are variable quantities that
depend on how fast the compression or expansion of the gas is achieved. Although
Wrev for an isothermal (reversible) compression
or expansion can be determined from the ideal gas equation and Qrev
for such a process can be determined from Wrev by invoking
the First Law, neither Wrev nor Qrev is
a difference in a property, or state function, of the gas. Wrev
for such a process can be determined from the ideal gas equation because, for
conditions of constant T, the state variables consist only of the intensive
property P and the extensive property V which correspond to one
another such that PV defines an energy having dimensions of mechanical
work (i.e., dimensions of force × distance).
As is evident from its exclusive dependence
on pressure and volume, free energy of concentration is a property of an ideal
gas. However, differences in this property between states can be calculated
only if the states have the same temperature. Although the free energy otherwise
has the advantage that it is clearly an actual property of the gas, differences
in this property between states at the same temperature are ordinarily reckoned
in terms of the thermal energy that would be transmitted between the gas and
its surroundings if the changes giving rise to the differences were to occur
reversibly. The achievement of this requires an additional extensive state property
that corresponds to the intensive state property T in such a way as to
define an energy having dimensions of thermal energy when multiplied by T.
In addition, since an isothermal change in the free energy would not involve
a net change in the actual energy of the gas and is assumed not to involve a
net change in the temperature of the surroundings, this extensive state property
must be limited to thermal energy that is produced or consumed at constant temperature.
In other words, (i) as for any other kind of energy, we must have a capacity
factor for thermal energy that corresponds to the intensity factor for this
kind of energy and (ii) the capacity factor in this particular case must
be limited to thermal energy that either does not contribute to temperature
or is otherwise hidden with respect to temperature.
The problem of recognizing and meeting this need was solved early in the history
of thermodynamics research by Clausius [5], who noted that,
despite the fact of the amount of thermal energy Qrev
being located in the surroundings rather than in the gas, Qrev /T
for an isothermal change in the state of an ideal gas corresponds to a change
in a property of the gas. Clausius named this property 'entropy' and represented
it with the symbol S. With respect to gases and other chemical substances,
the symbol S is now ordinarily used to express entropy as an intensive
quantity having the same dimensions as R, the universal gas constant
(i.e., dimensions of energy per mol per degree Kelvin). As a result,
nST defines an amount of energy just as does nRT of the ideal
gas equation, n being the number of mols and nS the extensive
component of the energy. However, whereas nRT refers to an amount of
pressure-volume work energy, nST refers to an amount of thermal energy
that is somehow hidden with respect to temperature. Of course if the thermal
energy of which entropy is the capacity factor were not hidden with respect
to temperature, the appropriate comparable capacity factor would be the molar
heat capacity of the substance.
The molar heat capacity of a substance refers to the amount of thermal energy
required to raise the temperature of one mol of the substance by one degree
Kelvin. The heat capacity of a gas can be determined unequivocally only under
conditions either of constant pressure or of constant volume, in which cases
the molar heat capacities are denoted by CP
and CV, respectively. According
to the classical kinetic theory of gases, CP
and CV for an ideal gas
would be constants having the values 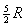 and
and
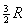 ,
respectively, regardless of the magnitudes of the state properties temperature,
pressure, and volume at which they are determined [6]. CP
exceeds CV by R because an amount of pressure-volume
work equivalent to R would be done on the surroundings if the heat capacity
were determined at constant pressure. Thus, only CV is a heat
capacity that refers exclusively to energy possessed by the gas.
,
respectively, regardless of the magnitudes of the state properties temperature,
pressure, and volume at which they are determined [6]. CP
exceeds CV by R because an amount of pressure-volume
work equivalent to R would be done on the surroundings if the heat capacity
were determined at constant pressure. Thus, only CV is a heat
capacity that refers exclusively to energy possessed by the gas.
As one might expect from its relationship
to free energy of concentration, entropy in general simply appears, never to
disappear, as free energy vanishes. As a result of the fact that its total amount
invariably increases, entropy is often said not to be conserved, which, of course,
is true in the sense that the amount is not a constant. Since the increase is
determined by the amount and quality of the actual energy that invariably appears
as high-quality actual energy and its associated free energy disappear, it seems
more appropriate and instructive to acknowledge instead that it is the free
energy (quality) of actual energy, or high-quality actual energy itself, that
is not conserved. Nevertheless, entropy has been universally adopted as the
principal index of free energy consumption, this despite the facts that the
quality of an energy is determined by the magnitude of its intensive component
[4] and that entropy, being, like volume, an extensive component
of an energy, is consequently relatively incomprehensible as an indicator of
energy degradation. However, as will be outlined below, entropy has an important
advantage over free energy in this respect in that it can be and has been universalized
such as to make possible a proper (practical) assessment of the quality of thermal
energy.
In this regard it is important to note that
the free energy of the intermediary thermal energy of a thermodynamic work process
mediated by an ideal gas would be subject to loss by two means depending on
whether the thermal energy moves spontaneously down a gradient of pressure by
convection, in which case the free energy that is subject to loss would be the
above-described free energy of concentration, or down a gradient of temperature
by conduction and/or radiation. If entropy is to be a universal capacity factor,
it must be capable of serving as the capacity factor for free energy that is
subject to loss by both these means. In the case of convection, the intensive
and extensive components of the energy are pressure and volume and the extensive
component can be readily expressed in terms of entropy. Thus, for an isothermal
(reversible) compression of an ideal gas, the increase in free energy of concentration
on a per mol basis can be represented either by -RT ln(V2 /V1)
or by -TDS, DS
being equivalent to R ln(V2 /V1), a negative
quantity in the case of compression, indicating a decrease in the entropy of
the gas. Of course the reason why the entropy of the gas would decrease in this
case is that the thermal energy on which the entropy is based would be located
in the surroundings rather than in the gas. Entropy is universalized by using
this volume-dependent entropy in a Carnot cycle to define an entropy in terms
of the thermal energy possessed by the gas, in which case the free energy that
is subject to loss would have temperature and heat capacity as its intensive
and extensive components. Since both CV and CP
would be constants, any changes in this 'derivative' entropy must necessarily
be expressed in terms of changes in the temperature and thermal energy of the
gas.
As is evident from the above-described properties of free energy of concentration,
the volume-dependent entropy of an ideal gas could change through either compression
or expansion without there being a change in the actual energy of the gas and,
in the case of expansion, could change in the absence of a net change in actual
energy of either the gas or its surroundings. Entropy being a state property,
it is also evident that the volume-dependent entropy would not depend on the
rate of change. In contrast, the temperature-dependent entropy, being based
on thermal energy possessed by the gas, would depend greatly on the rate of
change, a fact which can be readily seen by considering irreversibility in respect
to an adiabatic cycle of compression and expansion of an ideal gas (see Appendix).
In consequence of the different locations of the actual energies on which the
volume- and temperature-dependent entropies are based, one kind could increase
while the other decreases if the gas were to undergo a change in temperature
and thermal energy as well as in volume. In view of the marked differences between
the two entropies, it seems highly desirable to distinguish between them on
a regular basis more or less as done originally by Gurney [7]. In making this distinction in what follows, the kind of entropy
that depends only on the volume shall be referred to as entropy of concentration
to correspond with free energy of concentration. That this is appropriate can
be seen by noting that the concentration of a given amount of an ideal gas would
vary inversely as the volume regardless of the temperature and pressure and
that concentration can therefore be substituted for volume in the expression
R ln(V2 /V1) for a difference
in the volume-dependent entropy on a per mol basis simply by changing a sign.
The kind of entropy that would depend only on the temperature and thermal energy
of the gas shall be referred to as characteristic entropy, and the corresponding
kind of free energy shall be referred to as characteristic free energy. It is
important to note that these 'concentration' and 'characteristic' kinds of entropy
and free energy are very similar to but not entirely identical with the 'cratic'
and 'unitary' kinds defined by Gurney.
In contrast to changes in free energy of concentration, changes in characteristic
free energy are invariably accompanied by equivalent changes in the energies
recognized by the First Law. Since, as noted above, the only actual (First-Law)
energy of an ideal gas would be undirected translational kinetic energy, the
characteristic free energy of such a gas must necessarily be of the above-mentioned,
'potential' kind possessed by thermal energy (i.e., that recoverable
through work only at the expense of a decrease in the temperature of the energy
as its work potential is being realized). In view of the prediction of the ideal
gas equation that the size of a change in the free energy of concentration due
to a compression or an expansion between two states of concentration at the
same temperature can be determined on a per mol basis according to RT ln(V2 /V1) or RT ln(P2 /P1) and thus would be directly proportional
to temperature, it is evident that changes in free energy of concentration,
although not involving net changes in the characteristic free energy of the
gas, would require that the gas possess characteristic free energy if the changes
are to be finite, the characteristic free energy being that associated with
the thermal energy at the temperature of the two states. Of course the higher
the temperature, the greater the change in free energy of concentration for
a given change in concentration of the particles possessing the thermal energy
and free energy.
Changes in the characteristic free energy
are ordinarily reckoned in terms of work done on or by the gas during its compression
or expansion under adiabatic conditions (i.e., under conditions in which
transmissions of thermal energy between the gas and its surroundings cannot
occur). If this is to be achieved, one must have knowledge of the capacity factor
for thermal energy that determines temperature and refers only to energy possessed
by the gas. As noted above, CV, the molar heat capacity determined
under conditions of constant volume, meets this requirement and, for an ideal
gas, would not depend on the magnitudes of the state properties at which it
is determined. This being the case, the total energy for one mol of such a gas
would be given by CV T and finite changes in total energy on
a per mol basis by CV DT,
CV being a constant. Since reversible processes do not expend
free energy, CV DT for any reversible adiabatic compression or expansion
would be equivalent to the change in characteristic free energy as well as to
the change in total energy. Thus, if the quality of thermal energy were judged
on the basis of the predicted changes in the characteristic free energy of an
ideal gas, it would appear to be equivalent to that of nonthermal actual energy.
However, as outlined below, a proper assessment of the quality of thermal energy
in terms of changes in the properties of a gas can be achieved only by means
of the Carnot cycle.
The Carnot Cycle
Owing to the extensive use of gas heat engines to exploit the work potentials
of natural sources of nonthermal actual energy, an important concern of thermodynamics
is the availability (quality) of the work potential of thermal energy imparted
to a gas in the course of the gas undergoing a cycle of expansion and compression.
Since in a strictly adiabatic or strictly isothermal cyclic process, a gas cannot
do more work than is done on it, net conversion of thermal energy into nonthermal
actual energy is impossible in these cyclic processes. As pointed out originally
by Carnot [8], such a conversion can be achieved in a cyclic process only
by combining isothermal and adiabatic processes in an alternating sequence in
which the gas is allowed to undergo isothermal expansion with uptake of thermal
energy from a reservoir at a relatively high temperature, followed by adiabatic
expansion and isothermal compression with transmission of a smaller amount of
thermal energy from the gas to a reservoir at a relatively low temperature.
Net conversion is achieved exclusively as a result of the fact that a gas at
a relatively high temperature can do more work through isothermal expansion
than is required for isothermal compression by the same factor at a relatively
low temperature. If all four steps of the Carnot
cycle were conducted reversibly, none of the characteristic free energy of the
thermal energy absorbed isothermally by the gas at the relatively high temperature
and converted through work into the kind possessed by nonthermal actual energy
would be consumed. However, complete conversion of the free energy would be
possible only if the gas could undergo infinite expansion in the adiabatic expansion
phase of the cycle and the temperature of the relatively cold gas and reservoir
were at a temperature of absolute zero. Therefore, since available machines
are of limited size, and since the thermal sinks ordinarily available have temperatures
much higher than absolute zero, complete or nearly complete conversion of thermal
energy into energy equivalent in quality to gravitational potential energy is
far from being practical. Of course this is the basis for the limited potential
availability of the work potential of thermal energy.
As is evident from the Carnot cycle and the
above-noted fact that the quality of an energy is determined by the magnitude
of its intensive component, the practical quality of thermal energy is higher
as the temperature is higher. Determination of the quality by means of the Carnot
cycle requires knowledge of the relationship between temperature and volume
in the adiabatic steps. The required information is obtained by defining a characteristic
entropy in terms of entropy of concentration, which, as noted above, would be
a function only of volume and concentration for an ideal gas. Since entropy
of concentration is a capacity factor for thermal energy that is hidden with
respect to temperature, this characteristic entropy must be scaled to thermal
energy transmitted to or from the gas at constant temperature, a fact which
is relevant to the above-mentioned need to know the relationship between temperature
and volume in the adiabatic steps. The thermal energy to be evaluated as to
quality is that transmitted to the gas and converted into work isothermally
at the relatively high temperature. Since entropy refers to thermal energy,
it is the 'thermal fraction' of this thermal energy (i.e., the fraction
transmitted isothermally to the surroundings at the relatively low temperature)
to which changes in the characteristic entropy of the adiabatic steps must be
scaled. Of course the isothermal transmissions are assumed to be possible as
a result of the reservoirs being sufficiently large to be capable of yielding
and accommodating thermal energy without undergoing a change in temperature.
Owing to the fact that it would not be possible for the temperature and thermal
energy of an ideal gas to change independently of one another, a change in the
characteristic entropy can be expressed properly in terms of the temperature
and thermal energy only by means of the differential equation dSchar = dE/T
or its equivalent. Since dE = CV dT
and CV would be a constant,
this equation can be expressed in the forms dSchar = CV dT /T = CV d lnT
and integrated between two specific temperatures to yield DSchar = CV ln(T2 /T1).
The comparable expression R ln(V2 /V1)
for a finite change in the entropy of concentration on a per mol basis is obtained
essentially in the same manner. In this case, however, it is the pressure and
volume that would be incapable of changing independently of one another. In
consequence of this and of the fact that the volume must change if the entropy
is to change, a change in Sconc
can be expressed properly in terms of volume and pressure only by means of the
differential equation dSconc = PdV/T,
which can be modified by substituting RT /V for P to obtain
the equation in the integrable form dSconc = R d lnV.
Of course the scaling of DSchar
to DSconc
in the adiabatic steps is accomplished through the facts (i) that a net
change in entropy would not be possible in a reversible adiabatic process and
(ii) that, in consequence, the ratios of the initial and final temperatures
and volumes in the adiabatic steps of a reversible Carnot cycle would be constrained
to agree with one another according to the relationship: 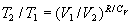 .
.
That DSchar
for the adiabatic steps is based on the thermal energy transmitted isothermally
to the surroundings at the relatively low temperature is evident from the facts
(i) that the maximum efficiency hmax
of a Carnot heat engine is equivalent to 1 - Tlow /Thigh
and (ii) that DSchar
for the adiabatic steps of the reversible cycle is therefore equivalent to ±CV ln(1 - hmax),
the positive and negative signs referring to the expansion and compression steps,
respectively. By noting that hmax
refers to the available (work) fraction of the thermal energy absorbed at Thigh ,
one can readily see that DSchar
refers to the unavailable (thermal) fraction. For any given finite value of
Thigh ,
the unavailable fraction is a linear function of Tlow
and decreases to zero as Tlow
approaches zero.
The Carnot cycle is very important in that it universalizes entropy and thereby
makes it possible to evaluate the practical quality of thermal energy and to
demonstrate by theoretical means for thermodynamic work processes in general
that, if such a process is to occur at a finite rate, free energy must be expended
and thermal energy must be produced in an amount equivalent to the amount of
free energy consumed, a fact which can be readily demonstrated by considering
irreversibility in respect to an adiabatic cycle of compression and expansion
of an ideal gas (see Appendix). In any particular
case, the fundamental process giving rise to the finite rate would be the net
conversion of relatively high-quality actual energy, both thermal and nonthermal,
into ambient thermal energy, the spontaneous and unidirectional nature of which
is the basis for the Second Law of Thermodynamics, which, unlike the First Law,
acknowledges the existence of free energy and says in effect that if thermodynamic
work is to be done at a finite rate, free energy must be expended. Also unlike
the First Law, the Second Law, owing to the fact that the individually mobile
particulate constituents of macroscopic amounts of matter at finite temperatures
vary widely as to translational kinetic energy, is a statistical law appropriate
for application only to macroscopic phenomena. This means that the Second Law
is obeyed only on average over time in processes at the microscopic level and
thus that conversions of ambient thermal energy into nonthermal actual energy
in chemically active substances can occur at the molecular level. Of significance
in this regard is the fact that translational thermal energy at the molecular
level is kinetic energy of the directed variety, a consequence of which is that
no distinction can be made between this form of actual energy and nonthermal
actual energy at the molecular level. Accordingly, energy transfer at the molecular
level occurs without expenditure of free energy, and irreversibility, like temperature
and pressure in respect to a gas, is a concept applicable only to macroscopic
phenomena.
Real Gases
As is well known, the heat capacities of real
gases increase with increase of temperature and correspond closely to those
predicted for an ideal gas over wide ranges of temperature and pressure only
for monatomic gases [9]. The temperature dependence is due
in large part to the fact that the translational and radiant forms of thermal
energy are capable of undergoing interconversion with actual energy associated
with quantized motions within molecules. The energies of these 'intramolecular'
motions are reckoned in terms of characteristic entropy and thus appear to be
generally viewed as being of a thermal nature, this despite the facts (i)
that the energies of some of the motions undergo oscillatory interconversions
with attractive and repulsive potential energies and (ii) that the motional
energies, being intramolecular, seem best viewed as being directed (nonrandom)
kinetic energies and thus of a nonthermal nature. On the other hand, since radiant
energy is not confined to molecules and is ordinarily unrestricted as to direction
of emission in a gas, any radiant energy emitted as a result of the motions
would clearly qualify as thermal energy.
In accord with their being nonthermal, the
energies associated with the intramolecular motions apparently do not contribute
to temperature. Such is consistent with the facts (i) that the absolute
and thermodynamic scales of temperature are based on the properties of an ideal
gas and (ii) that an ideal gas is assumed to possess actual energy only
of the translational kinetic kind. It may be noted that, owing to the discontinuous
nature of the intramolecular motions in respect to change of temperature, such
must be assumed for validity of the widely accepted, sweeping generalization
concerning the reversible Carnot cycle that the ratio of the amount of thermal
energy absorbed isothermally at Thigh to the amount rejected
isothermally at Tlow would have the same value regardless
of the nature of the working substance.
Despite their apparent nonthermal nature,
the energies associated with the intramolecular motions appear to be recoverable
only as thermal energy at the temperature of their reversible production and
thus appear to differ markedly from the kind of nonthermal energy that is capable
of converting spontaneously into thermal energy of quality exceeding that of
the ambient thermal energy. Accordingly, their elicitation is associated with
diminution of molecular stability [10] and might be expected
thereby more likely to diminish than to enhance chemical potential energy of
the kind that possesses characteristic free energy. This kind of energy shall
henceforth be referred to as 'characteristic chemical potential energy', a distinction
made necessary by the fact that free energy of concentration is ordinarily treated
as if it were chemical potential energy, a practice which of course is valid
when properly used but which ignores the intrinsically mechanical nature of
the concentration-dependent free energy and tends to elicit confusion as to
the nature of characteristic chemical potential energy, particularly when used
in reference to an ideal gas.
Although, as noted above, the energies associated
with the intramolecular motions also differ appreciably from what is ordinarily
considered to be thermal energy, in view of the current practice of accounting
for them appropriately in terms of characteristic entropy and of entropy being
the capacity factor for thermal energy that is hidden with respect to temperature,
it seems appropriate and best for practical purposes to view the energies as
being latent forms of thermal energy of quality determined by the temperature
at which they could be reversibly produced. By making this distinction we imply
that thermal energy that is not latent refers to the kind that determines temperature.
Since what has been referred to above as the radiant form of thermal energy
is actually electromagnetic energy having frequency n
as its intensive component and nh as its extensive component, n
being any whole positive number and h Planck's constant, and can be said
to be thermal energy only in the sense that it is capable of transmitting thermal
energy and to have a temperature only in virtue of the fact that it has a certain
distribution as to quality and concentration of photons of energy hn as given by Planck's Law of Heat Radiation when in
equilibrium with matter at a particular temperature [11], this 'nonlatent' thermal energy may be considered to consist
exclusively of the translational kinetic kind. However, since electromagnetic
energy is readily detectable and transmissible as such, when viewed as being
thermal energy it must in some sense also be viewed as being a nonlatent variety,
particularly in respect to transmission of thermal energy.
The intramolecular motions include rotations
of entire molecules and various rotations, librations, and vibrations of molecular
constituents, all of which are known to be quantized through the occurrence
of temperature-specific changes in heat capacity and in absorption and emission
of characteristic radiant energy [10, 12].
Of course the possibilities for these kinds of motion are greater, the greater
the complexity of the molecules and the weaker and more flexible the bonds between
the constituent atoms. In view of the fact that the intramolecular motions can
be elicited through inelastic collisions between molecules, these motions must
be capable of converting into the translational motions that result in the collisions
and, under conditions of constant temperature, must tend to be at equilibrium
with the translational motions; otherwise, contrary to Planck's Law and the
Zeroth Law of Thermodynamics, there would likely be a temperature differential
between the translational and radiant forms of thermal energy at equilibrium.
Due to the existence of net attractive forces between the individually mobile
particulate constituents of macroscopic amounts of real matter, thermal energy
can also disappear and appear with increase and decrease of temperature through
dissociation and association reactions that increase and decrease the number
of particles whose motions contribute to and thereby determine temperature.
This phenomenon can be explained on the basis of the very successful prediction
of the equipartition principle of the classical kinetic theory of gases that,
at any particular temperature, individually mobile particles differing as to
mass, composition, and other properties will possess on average the same amount
of translational kinetic energy, the amount being equivalent to 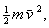 m being the mass and
m being the mass and 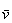 the
average velocity [9, 12, 13].
According to this prediction, if the molecules of a gas at a particular temperature
were sufficiently attracted to one another that some of the molecules could
expend attractive binding energy by binding to one another at that temperature,
increasing the temperature would result in mechanically induced dissociations
of bound molecules and in a portion of the thermal energy added to the gas for
the purpose of increasing its temperature being expended to elevate the translational
kinetic energies of newly formed particles to average values consistent with
the existing temperature. Since the formation of the additional particles would
be accompanied by the appearance of attractive forces and thus also of attractive
binding energy, it seems appropriate to view the dissociations of the attractively
bound particles as constituting conversions of translational kinetic (mechanical)
energy into attractive binding energy. Also, since it is unlikely that attractive
binding energy thus generated could be recovered as an energy of quality higher
than the thermal energy required for its reversible production, and since the
attractive forces would extend beyond the bounds of the particles possessing
them, it seems appropriate to view the attractive binding energy as being an
extramolecular kind of latent thermal energy of quality determined by the temperature
at which it could be reversibly produced. As will be pointed out below, one
could reasonably expect the attractive binding energy to have the quality of
characteristic chemical potential energy only if the gas were supercooled with
respect to thermochemical equilibrium.
the
average velocity [9, 12, 13].
According to this prediction, if the molecules of a gas at a particular temperature
were sufficiently attracted to one another that some of the molecules could
expend attractive binding energy by binding to one another at that temperature,
increasing the temperature would result in mechanically induced dissociations
of bound molecules and in a portion of the thermal energy added to the gas for
the purpose of increasing its temperature being expended to elevate the translational
kinetic energies of newly formed particles to average values consistent with
the existing temperature. Since the formation of the additional particles would
be accompanied by the appearance of attractive forces and thus also of attractive
binding energy, it seems appropriate to view the dissociations of the attractively
bound particles as constituting conversions of translational kinetic (mechanical)
energy into attractive binding energy. Also, since it is unlikely that attractive
binding energy thus generated could be recovered as an energy of quality higher
than the thermal energy required for its reversible production, and since the
attractive forces would extend beyond the bounds of the particles possessing
them, it seems appropriate to view the attractive binding energy as being an
extramolecular kind of latent thermal energy of quality determined by the temperature
at which it could be reversibly produced. As will be pointed out below, one
could reasonably expect the attractive binding energy to have the quality of
characteristic chemical potential energy only if the gas were supercooled with
respect to thermochemical equilibrium.
Since associations of molecules through mutual
attractions are likely to result in some hindrance of intramolecular motions,
changes in latent thermal energy of the extramolecular kind at temperatures
sufficiently high for elicitation of intramolecular motions are likely to be
accompanied by changes in latent thermal energy of the intramolecular kind in
the same direction. In contrast to the intramolecular kind, the extramolecular
kind can undergo change in response to change not only of temperature, but also
of pressure and thus of concentration. Both kinds can change under conditions
of constant temperature and pressure in chemical reactions and in first-order
phase transitions, and, since both are based on system energies having the quality
of thermal energy, changes in their amounts under these conditions are actually
and best accounted for in terms of characteristic entropy.
As indicated above, the prediction of the
classical kinetic theory of gases that CV for an ideal gas
would be a true constant implies that the amount of thermal energy possessed
by a given amount of such a gas would be directly proportional to temperature.
This in turn implies that an ideal gas can be considered to provide linear absolute
scales for actual energy and free energy as well as for temperature. Although
based on predicted properties of a fictitious gas that differs appreciably from
real gases in that its particulate constituents possess only translational kinetic
energy, these scales are very important in that they are commonly used with
remarkable success as a framework for characterization of the thermodynamic
properties of all real substances, a fact which accords with the universality
of the universal gas constant R. As will in effect be suggested below,
the widespread success of the ideal gas model is likely due in large part to
the above-noted prediction of the equipartition principle being applicable to
liquids and solids as well as to gases and to temperature in liquids and solids
being determined by the average kinetic energies of individually mobile particles
consisting of clusters of attractively bound molecules, the average size of
which tends to increase with decrease of temperature.
Thermodynamics of Liquids and Solids
All naturally occurring gases possess net
attractive forces and in consequence undergo condensations to form liquids and
solids as temperature is decreased. An important but rarely asked question is:
What determines temperature in these condensed phases? This question must be
asked and answered correctly if we are to understand how it is possible that
equilibrium differences in concentration between the reactants and products
of a chemical reaction conducted in solution can serve to measure differences
in characteristic chemical potential energy between the reactants and products
at various equilibrium temperatures.
Since the particulate constituents of liquids
can flow and thus must be sufficiently free to undergo translational motions,
we can attribute temperature in this case to the same kind of motions that determine
temperature in gases. Since the particulate constituents are held in the liquid
state by attractive (cohesive) forces possessed by the particles, one might
expect the translational motions of the particles to be hindered in respect
to intensity of translational motions and thus in respect to ability to contribute
to and thereby determine temperature. However, in view of (i) the likelihood
that temperature is determined in both liquids and gases by the average intensity
of the actual translational motions of the constituent individually mobile
particles and of (ii) the above-noted prediction of the equipartition
principle that, at any particular temperature, the individually mobile particulate
constituents of all gases will possess on average over time the same amount
of translational kinetic energy, it seems likely that the average translational
kinetic energy possessed by the particulate constituents of any liquid would
tend to be the same as that for any gas if the liquid and gas were at the same
temperature. If such were not the case, it would be very difficult to understand,
among other things, the physical bases for the latent thermal energies and for
the common observation that the mechanical thermodynamic properties of substances
in dilute (ideal) solution do not differ appreciably from those expected of
an ideal gas despite the individual molecules of the substances differing greatly
as to such properties as chemical composition, size, net charge, and affinity
for the solvent.
As is well known, condensations of gases into
liquids and of the liquids into solids under conditions of constant pressure
can occur very nearly reversibly at constant temperature with productions of
amounts of thermal energy that greatly exceed the amounts of pressure-volume
work done simultaneously. It is important to inquire as to where the excess
thermal energy comes from in these processes. Since the condensations occur
as a result of there being attractive forces between the particulate constituents,
it must necessarily come primarily from conversions of attractive binding energy
into thermal energy as the particles associate in the course of the condensations.
In view of this and of the likelihood that the average translational kinetic
energies of the individually mobile particulate constituents of at least the
liquid and gas phases of any substance would be the same if the phases were
at the same temperature, it seems likely that such conversions occur through
associations of particles to form larger and thus fewer particles possessing
on average the same amount of translational kinetic energy as the particles
undergoing the associations. Thus it seems appropriate to view the attractive
binding energy that undergoes conversion into thermal energy in phase transitions
of the above sort to be latent thermal energy of the above-described extramolecular
kind.
As is particularly well known, thermally induced
transitions of water from solid to liquid and from liquid to vapor at normal
(atmospheric) pressures involve large conversions of thermal energy into latent
thermal energy. It is also well known that some of the latent thermal energy
is of the intramolecular kind and that the conversions are capable of occurring
at constant temperature with little consumption of free energy when the nonlatent
thermal energy derives from ambient thermal energy [14].
By linking reversible production of latent thermal energy in these processes
to isothermal (reversible) compression of an ideal gas, we can readily see that
both the intramolecular and the extramolecular kinds of latent thermal energy
must in fact be viewed as being equivalent in quality to ambient thermal energy
at the temperature of their reversible production. Consider, for example, a
Figure 1 system in which the temperature of the thermal reservoir
is at the melting temperature of ice and is maintained constant solely by interconversions
between ice and liquid water at constant external pressure. If the gas in such
a system were compressed reversibly, thermal energy would be produced isothermally
in the gas and consumed isothermally in the reservoir through reversible conversion
of ice into liquid water. Since all of the free energy of the nonthermal actual
energy imparted to the gas would be retained by the gas and could be conserved
only through reversal of the above process, it would be necessary to consider
any latent thermal energy produced as a result of translational thermal energy
from the gas converting ice into liquid water to be equivalent in quality to
ambient thermal energy at the melting temperature of the ice.
The above observations being correct, one
could reasonably expect the number of individually mobile particles in a liquid
to increase as the temperature is increased. Since the increase would necessarily
occur through dissociations of attractively bound particles, one could reasonably
expect the heat capacities of liquids to be augmented by conversions of thermal
energy into attractive binding energy as the temperatures of the liquids are
increased for the purpose of measuring the heat capacities. That these expectations
are consistent with what is observed experimentally in this regard is particularly
clear in the case of water.
Owing to the molecules of water as compared to those of most other common solvents
having particularly strong tendencies to associate with one another through
their capacities to serve both as a double donor and a double acceptor of hydrogen
bonds, the energy changes in the gas  liquid
and liquid
liquid
and liquid  solid
transitions of water are particularly large. The molar heat capacity of the
liquid at constant atmospheric pressure is also particularly large and is close
to twice that of the solid near the normal freezing point and to twice that
of the vapor near the normal boiling point [15]. The above-noted
expectations are clearly consistent with these experimental findings and with
numerous experimental and theoretical observations suggesting that the particulate
constituents of liquid water consist largely of labile clusters of molecules,
the concentration and average size of which depend on temperature and pressure
[15-18].
solid
transitions of water are particularly large. The molar heat capacity of the
liquid at constant atmospheric pressure is also particularly large and is close
to twice that of the solid near the normal freezing point and to twice that
of the vapor near the normal boiling point [15]. The above-noted
expectations are clearly consistent with these experimental findings and with
numerous experimental and theoretical observations suggesting that the particulate
constituents of liquid water consist largely of labile clusters of molecules,
the concentration and average size of which depend on temperature and pressure
[15-18].
For there to be consistency with the above observations, it is necessary to
suppose that, for solids, temperature and to a large extent also heat capacity
are determined by undirected translational (vibrational) motions of clusters
of molecules (atoms in the case of atomic solids) about mean fixed positions
and that the average size of these clusters tend to increase with decrease of
temperature, the increase in average size being accompanied by conversion of
kinetic and potential (attractive + repulsive) vibrational energy into thermal
energy. In view of the fact that the average vibrational frequency of the clusters
would decrease as the average size (mass) of the clusters increases and of experimental
observations suggesting that the heat capacities of all naturally occurring
substances tend to approach zero as temperature approaches zero, in considering
this to be the case for a large chunk of a solid maintained close to thermochemical
equilibrium, it would seem necessary to assume that the number and average vibrational
frequency of the clusters would decrease to unity and zero, respectively, as
the temperature of the solid is decreased to zero. This and the further assumption
that the atoms or molecules of the individual clusters vibrate coherently and
consequently emit photons as clusters of indistinguishable photons being correct,
one could think of the clusters as being Bose-Einstein condensates of a sort
and of the solid as being a Bose-Einstein ideal gas in which the number of particles
is not conserved more or less as the Bose-Einstein quantum statistical method
of deriving Planck's Law of Heat Radiation predicts for distinguishable clusters
of indistinguishable photons in equilibrium with a blackbody (see below). Doing
so would be consistent with the generally accepted Bose-Einstein quantum gas,
'quasiparticle' (phonon) interpretation of lattice vibrations in crystalline
solids [11, 19, 20]
and thus with the apparent wave-particle duality of matter in this case. Doing
so would also be consistent with experimental observations [21,
22] indicating that water ice at subfreezing temperatures
sublimates (evaporates) in the form of clusters of molecules.
In 1913 prior to the general acceptance of
the quantum hypothesis demanded by the extremely close agreement between experimental
observations and the equation now known as Planck's Law of Heat Radiation, Benedicks
[23] presented an 'agglomeration hypothesis' which appears
to be generally consistent with the above notions concerning what determines
temperature and heat capacity in solids. Thus, Benedicks pointed out that Planck's
Law can be derived on the basis of the assumptions (i) that the atoms
or molecules of solids coalesce through cohesive forces to form clusters of
increasing size as temperature is decreased and (ii) that the thermal
energy partitions among the clusters in accordance with the equipartition principle.
He also pointed to the possibility of explaining the observed independence of
the frequency spectrum of cavity (equilibrium blackbody) radiation on the nature
of the solid by taking into account the facts (i) that the average mass
of the clusters at a particular temperature would be greater as the cohesive
force between the constituent atoms or molecules is greater and (ii)
that, since the average vibrational frequency of the clusters would be lower
as the average mass of the clusters is greater and higher as the cohesive force
between the clusters is greater, differences among solids as to cohesive force
would tend to cancel in respect to the vibrational frequency spectrum of the
clusters at a particular temperature and thus also in respect to the frequency
spectrum of the radiant energy emitted and absorbed by the clusters at that
temperature.
In 1915 A. H. Compton [24]
tested the agglomeration hypothesis as to utility in accounting for experimentally
observed relationships between temperature and heat capacity in simple solids.
Using Maxwell's Distribution Law in conjunction with the agglomeration hypothesis,
he came up with a very simple equation which he judged to be at least equally
as accurate as the much more complex but now generally accepted equation developed
earlier by Debye [25] on the basis of the quantum hypothesis
using arbitrarily the assumption that vibrational frequencies at low temperatures
are limited to the low values that one might expect to be characteristic of
large clusters of atoms. Compton [26] also compared the agglomeration and quantum hypotheses as
to utility in accounting for the observed inverse relationship between temperature
and thermal conductivity in solids and found the agglomeration hypothesis to
be clearly superior in this case.
Despite its remarkable successes, the agglomeration
hypothesis was largely abandoned, presumably as a result of Compton turning
his attention to his well-known studies on interactions between X-rays and matter,
which resulted in the general acceptance of the quantum hypothesis, and to the
textbook view [27] that if the agglomeration hypothesis
were valid, one could expect solids to be incompressible at temperatures approaching
absolute zero, a view which has persisted into modern times [28] despite the fact that it was based on the faulty notion
that atoms are incompressible (hard) spheres (see [29]).
Nevertheless, although not acknowledged or recognized, the main features of
the agglomeration hypothesis were used in the subsequent development of quantum
mechanics, a fact which is particularly evident in the case of wave mechanics.
Thus, Bose-Einstein (quantum) statistics become essentially identical with Maxwell-Boltzmann
(ideal-gas) statistics as temperature is increased and assumes a condensation
(degeneration) of distinguishable particles (parcels) of energy into distinguishable
clusters (cells) of indistinguishable particles of energy as temperature is
decreased [9]. Also, de Broglie's fundamental ideas
on wave-particle duality, which, in conjunction with Bose-Einstein statistics
led Schrödinger immediately to his wave mechanics [30],
originated with the realization that derivation of Planck's Law by considering
light quanta to be an ideal gas of photons ("atoms of light") requires the assumption
that blackbody radiation other than that at the extreme high-frequency end of
the observable spectrum consists of agglomerations of photons that move coherently
[31-33]. Since the frequency at the high-frequency end
of the observable spectrum increases endlessly with increase of temperature,
this assumption and the essentially identical one at the heart of Bose-Einstein
statistics being correct, most if not all 'conventional photons' would be clusters
of photons having the same frequency and we could imagine a rational explanation
for the peculiar fact of quantum mechanics that what appear to be single photons
can appear to split and interfere with themselves in a wave-like manner. However,
the explanation would not be of a sort that would seem likely to account for
the considerably more peculiar, closely related fact that electrons, neutrons,
and other genuine particles in what seem highly likely to be genuine (indivisible)
single-particle states also can appear to split and interfere with themselves
in a wave-like manner (see, e.g., [34, 35]).
That temperature and heat capacity in solids
are actually and strongly linked to the average size of actual particles consisting
of clusters of atoms or molecules is suggested by the results of numerous relatively
recent studies on the thermodynamic properties of 'nanosolids' obtained by reducing
normal (bulk) solids at temperatures far below their melting points to particles
having diameters of a few nanometers and then lightly compacting the particles
to form pellets that can be easily handled and compared with the bulk materials
(for comprehensive reviews, see [36, 37]). In general, the thermodynamic
properties of solids thus modified have been found to differ from the normal
such as to suggest that the temperature has in effect been greatly increased.
Thus, melting temperatures, at least of individual particles, are greatly decreased
[38], enthalpies [39], entropies [36],
heat capacities [36, 39], and vapor pressures [40] are greatly
increased, and heat capacities appear not to approach zero as temperature approaches
zero [36, 41]. Most of these changes
either have been or can be adequately explained in terms of the associated large
increases in surface area, surface attractive binding energy, and number of
particles that are individually mobile under conditions of temperature where
the attractive binding energy has the quality of characteristic chemical potential
energy rather than of latent thermal energy (see below). The observed persistence
of heat capacity in lightly compacted nanosolids at temperatures very close
to zero is consistent with the theoretical findings of Jura and Pitzer [42]
predicting that the heat capacities of solids consisting of unconsolidated 'nano-sized'
particles will be detectably large at temperatures closely approximating absolute
zero as a result of the particles being capable of undergoing thermally induced
translational and rotational motions at such temperatures despite the particles
possessing an abundance of attractive binding energy.
Third Law of Thermodynamics
According to the first of two extant versions of the Third Law that are relevant
here, the entropy change associated with any isothermal (reversible) process
will approach zero as temperature approaches zero [43, 44]. It is important to inquire as
to the nature of the entropy to which this version of the law refers. Since
it concerns only processes occurring at particular temperatures, characteristic
entropy of the kind that can change only as a result of a change in temperature
can be immediately ruled out, thereby avoiding the uncertainty associated with
the prediction of the kinetic theory of gases that a reversible decrease in
the temperature of an ideal gas to zero in a Carnot cycle would be accompanied
by a decrease in characteristic entropy to -∞, a result
necessitated by the fact that achievement of the condition T = 0
in the cycle would require increases in the volume and entropy of concentration
to +∞. On the other hand, entropy of concentration
can undergo change at constant temperature and can do so without necessarily
requiring a change in volume, a fact which can be readily seen by considering
chemical reactions of the sort: A  P + Q
(i.e., reactions in which there is a difference in mol number between
the reactants and products in the stoichiometric equation). As will be outlined
below, at least for reactions occurring in solution virtually at constant volume,
a change in entropy of concentration due to a change in the number of particles
in such a system maintained at constant temperature will be accompanied by a
change in the characteristic entropy of the system and the thermal energy on
which the characteristic entropy is based will be indistinguishable from the
above-described extramolecular latent kind. That a change in entropy of concentration
would conform to the above version of the Third Law can be seen by noting that
changes in entropy of concentration at a particular temperature serve to represent
changes in free energy of concentration and that, since free energy of concentration
depends on the existence and density of translational kinetic energy and therefore
can be expected to be zero at T = 0, a change in entropy of
concentration would not be possible at T = 0.
P + Q
(i.e., reactions in which there is a difference in mol number between
the reactants and products in the stoichiometric equation). As will be outlined
below, at least for reactions occurring in solution virtually at constant volume,
a change in entropy of concentration due to a change in the number of particles
in such a system maintained at constant temperature will be accompanied by a
change in the characteristic entropy of the system and the thermal energy on
which the characteristic entropy is based will be indistinguishable from the
above-described extramolecular latent kind. That a change in entropy of concentration
would conform to the above version of the Third Law can be seen by noting that
changes in entropy of concentration at a particular temperature serve to represent
changes in free energy of concentration and that, since free energy of concentration
depends on the existence and density of translational kinetic energy and therefore
can be expected to be zero at T = 0, a change in entropy of
concentration would not be possible at T = 0.
The same can be said of entropy of mixing,
the nature of which can readily be seen to be identical with that of entropy
of concentration by considering a spontaneous mixing of two distinguishable
ideal gases at the same temperature and initial pressure. A change in entropy
of mixing in this case is given by the sum of the increases in the entropies
of concentration of the individual gases as each diffuses (expands) into the
space occupied by the other. According to this, entropy of mixing serves to
account for the free energy of concentration that could be used for conversion
of ambient thermal energy into nonthermal actual energy if there were a means
by which the diffusion of the substances undergoing spontaneous mixing could
be harnessed individually to do work, a possible means in the above case being
an apparatus consisting of a cylinder having two chambers separated initially
by two opposed semipermeable pistons, the piston nearest each gas in its pure
form being permeable only to that gas. In the absence of such means, the mixing
would occur without a net change in actual energy, just as would be the case
if the individual gases were expanding into a vacuum.
Presumably, the principal kind of entropy
to which the above version of the Third Law is ordinarily meant to apply is
the characteristic entropy associated with the kinds of energy referred to above
as intramolecular and extramolecular latent thermal energy. As was noted, these
forms of energy do not contribute to temperature and can undergo change in processes
occurring at constant temperature. Since, at any particular finite temperature,
they tend to be at equilibrium with the transmissible (nonlatent) forms of thermal
energy, they can be expected to disappear as temperature approaches zero if
sufficient time is allowed for them to do so.
Owing to the tendency of the latent thermal energies to equilibrate with the
transmissible forms, the Third Law is widely interpreted to mean that the characteristic
entropies of individual substances approach zero as temperature approaches zero.
Although the version thus obtained has proved to be very useful for practical
purposes and is consistent with experimental observations suggesting that the
heat capacities of all naturally occurring substances in macroscopic amounts
maintained close to thermochemical equilibrium will decrease to zero as the
temperature is decreased to zero, it is considered not to be generally correct
as a result of the possibility for the characteristic entropy associated with
the extramolecular kind of latent thermal energy to become 'frozen in' as the
temperature is decreased. As implied above, the extramolecular kind of latent
thermal energy is clearly nonthermal, is possessed by all naturally occurring
substances at finite temperatures, and consists of attractive binding energy
that is subject to loss upon temperature reduction through stabilization of
neutralizing attractive binding interactions among the particulate constituents
whose undirected motions contribute to and thereby determine temperature. In
this particular case, latent thermal energy and its associated entropy can become
'frozen in' as a result of free molecules condensing during temperature reduction
to form stable solids in which the molecules are not arranged as well as they
could be for maximum conversion of the binding energy into the radiant and translational
forms of thermal energy, which, in contrast to the binding energy, are transmissible
as such and subject to direct removal through reduction of the ambient temperature.
For reasons of this sort, the Third Law, as it is applied to individual substances,
is generally considered to be applicable in a strict sense only to pure substances
in perfect crystalline states [45].
Thermodynamics of Biochemical Reactions
We shall be concerned here primarily with
the very simple, first-order, uncatalyzed reaction:
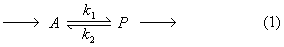
in which A and P are reactant and product in ideal
solution at constant temperature and pressure, k1 and k2
are the forward and backward rate constants, and
the arrows preceding the reactant and following the product are meant to indicate
that a and p, the concentrations of A and P, are maintained constant
as a result of the reaction being in a long sequence of reactions of the sort
that one might expect to find in living systems. Thus, Reaction (1) as presented
is considered to be a stationary-state reaction in an open system of more or
less constant volume and to have a net velocity in the direction indicated regardless
of whether the equilibrium constant is favorable or unfavorable. Although the
system as presented is considered to be open and therefore relevant primarily
to biological systems, in most of what follows it will be considered to be closed
(i.e., capable of exchanging only thermal energy with the surroundings).
Regardless of the nature of the energy from
which it derives, the available work potential of a chemical substance at a
particular combination of temperature and pressure is referred to either as
the Gibbs energy or the Gibbs free energy. When expressed as an intensive property
in terms of energy per mol, the Gibbs energy is referred to either as the Gibbs
potential or the chemical potential. Differences in this potential between states
of a substance and between substances in well-defined states are denoted by
DG. Since it is the difference in the intensive properties
of a system that determines the driving force for change, the driving force
of Reaction (1) as presented may be considered to be this difference between
A and P as given by the van't Hoff reaction isotherm:
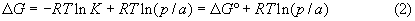
Here K is the equilibrium constant,
DG° is the so-called 'standard' difference in characteristic
Gibbs free energy between one mol of A and one mol of P, and DG
is the amount of Gibbs free energy consumed in the
net conversion of one mol of A into one mol of P under a specific set of conditions
of temperature, pressure, a, and p, the temperature, pressure,
and other environmental conditions being the same as those used in the determination
of K and DG°. Since DG as a driving force always refers to free energy that
is consumed, it is usually presented as -DG (i.e., as a positive quantity) when used in
this sense.
The van't Hoff reaction isotherm serves to
acknowledge that a particulate substance that is capable of undergoing chemical
change possesses (i) an available work potential that is independent
of its concentration and is characteristic of the substance in a given environment,
and (ii) an available work potential that depends only on the number
of individually mobile particles per unit volume (i.e., only on particle
concentration). The isotherm, as it applies to Reaction (1), can be derived
from the ideal gas equation on the basis of the observation by van't Hoff [1]
that the free energy of a solute in dilute (ideal) solution depends on the osmotic
pressure of the solute just as the free energy of an ideal gas would depend
on its pressure. Since, for an isothermal change in the state of such a gas,
the pressure and concentration would vary in direct proportion to one another,
the expression for a same-temperature difference in free energy of concentration
in terms of pressure obtained from the ideal gas equation may be modified by
substituting concentration for pressure. The expression then can be used as
a measuring device to determine differences in characteristic chemical potential
energy between the reactants and products of chemical reactions conducted in
solution at constant temperature and pressure from equilibrium differences in
translational kinetic energy density (i.e., from equilibrium differences
in free energy of concentration). The means by which this is ordinarily achieved
in respect to biochemical thermodynamics, in which case the solutions are usually
considered to be ideal, is outlined immediately below.
Determination of DG° by the Equilibrium Method
At a given temperature and pressure, the Gibbs potential G of a chemical
substance in ideal solution is equivalent to RT ln c + C,
where c is the concentration and C is an unknown constant. As is evident
from the fact that it can be expressed in the form V = Ae-G/RT,
where V = 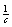 = molar volume and A = eC/RT,
this foundational relationship of the classical approach to chemical thermodynamics
has much in common with those of the Maxwell-Boltzmann statistical approach.
In consequence of C being unknown, G must be determined in relation to
a reference Gibbs potential G° and its concentration component RT ln c°,
which by convention are subtracted from G and RT ln c.
Thus, G - G° = RT ln c - RT ln c°
and G = G° + RT ln(c/c°).
By convention, the difference in Gibbs potential between a reactant and a product,
such as A and P of Reaction (1), is obtained by subtracting the Gibbs potential
of the reactant from that of the product. Thus, for Reaction (1):
= molar volume and A = eC/RT,
this foundational relationship of the classical approach to chemical thermodynamics
has much in common with those of the Maxwell-Boltzmann statistical approach.
In consequence of C being unknown, G must be determined in relation to
a reference Gibbs potential G° and its concentration component RT ln c°,
which by convention are subtracted from G and RT ln c.
Thus, G - G° = RT ln c - RT ln c°
and G = G° + RT ln(c/c°).
By convention, the difference in Gibbs potential between a reactant and a product,
such as A and P of Reaction (1), is obtained by subtracting the Gibbs potential
of the reactant from that of the product. Thus, for Reaction (1):
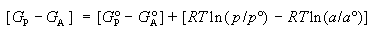
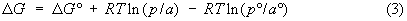
As may be seen, acquisition of the reaction isotherm in the form (Equation 2) in which it is usually presented and used in
respect to Reaction (1) requires that we get rid of the RT ln( p°/a°)
term in Equation (3). Since the logarithm of unity is zero, this can be done
very easily in this particular case by assuming a° = p°.
By making this assumption, we eliminate the free energy of concentration (mechanical)
component of DG° and thereby assume DG°
to be equivalent to the difference only of the characteristic (chemical) kind
of Gibbs free energy between one mol of A and one mol of P.
Since A and P are assumed to be in the same
environment, the difference in characteristic free energy on a per mol basis
between them in their common environment could be determined in a closed system
from their relative concentrations when the reaction is at equilibrium, in which
case DG would be zero, p/a would be equivalent
to the equilibrium constant of the reaction, and DG° would be equivalent to -RT lnK. This
assumes of course that the equilibrium constant is not so large or small as
to preclude measurement of a or p.
As may be seen by including the reference
concentrations in the reaction isotherm, the equilibrium constant is invariably
a dimensionless quantity. Such is required if the logarithm of an equilibrium
constant is to make sense [46]. Inclusion of the reference
concentrations in the isotherm has the additional advantage of allowing one
to see clearly how to handle a reactant or product that is maintained at constant
concentration or virtually so in the course of the reaction. Thus for such a
reactant or product, one need only specify the constant concentration to be
the reference concentration, in which case the reactant or product would be
eliminated from the isotherm. Of course any special condition of this nature
would limit the equilibrium constant to that condition and it would thus be
necessary to specify the limitation in presenting the equilibrium constant if
not done so by convention.
For reactions having multiple reactants and products, one's choice of reference
concentrations is arbitrary within the restriction that the sum of the reference
free energies of concentration on a per mol basis be the same for the reactants
as for the products. Note that, since Gibbs energies are expressed on a per
mol basis for each mol of each reactant and product specified in the stoichiometric
equation for the reaction, reactions having more than or less than one mol of
a particular reactant or product in their stoichiometric equation must be treated
such that DG° for the reactions 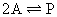 and
and
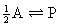 ,
for example, be equivalent to
,
for example, be equivalent to 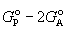 and
and
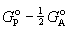 ,
respectively. Note also that, regardless of the complexity of the reaction,
DG° is expressed in units of energy per mol. For reactions
other than the very simple Reaction (1), one might
ask: energy per mol of what? By taking into account the ideal-gas and mechanical
(Figure 1) origins of the above method and using reciprocal
concentrations (i.e., molar volumes) rather than concentrations, one
can readily see that the answer must be: per mol of individually mobile particles.
In other words, one can readily see that the characteristic Gibbs energy of
each mol or fraction of a mol of each reactant and product specified in the
stoichiometric equation is measured in terms of the density of the translational
kinetic energy possessed by one mol of individually mobile particles at the
temperature of the reaction system. Thus, the 'per mol' may be interpreted to
indicate simply that the stoichiometric coefficients refer to mols rather than
to molecules.
,
respectively. Note also that, regardless of the complexity of the reaction,
DG° is expressed in units of energy per mol. For reactions
other than the very simple Reaction (1), one might
ask: energy per mol of what? By taking into account the ideal-gas and mechanical
(Figure 1) origins of the above method and using reciprocal
concentrations (i.e., molar volumes) rather than concentrations, one
can readily see that the answer must be: per mol of individually mobile particles.
In other words, one can readily see that the characteristic Gibbs energy of
each mol or fraction of a mol of each reactant and product specified in the
stoichiometric equation is measured in terms of the density of the translational
kinetic energy possessed by one mol of individually mobile particles at the
temperature of the reaction system. Thus, the 'per mol' may be interpreted to
indicate simply that the stoichiometric coefficients refer to mols rather than
to molecules.
The conventional way of eliminating the reference
term is to assume for each kind of reactant and product in the stoichiometric
equation a hypothetical 'standard state' concentration of unity in whatever
units of concentration are employed. This state is 'hypothetical' in that the
reactants and products are assumed to have properties identical with those they
would have if they were in dilute (ideal) solution, in which case the requirement
that concentrations be in terms of numbers of individually mobile particles
per unit volume is likely to be met. Although this convention invariably abolishes
the reference concentration term and, since the logarithm of unity to any power
is zero, is the most logical and efficient means of doing so, the fact of its
use is often stated in such a way as to give the very confusing impression that
the reactants and products must be at a concentration of one molar or one molal,
for example, if the value of DG° is to be correct, implying incorrectly that its use
has a purpose in addition to specification of dimensional units and elimination
of the reference term.
Nature of Characteristic Chemical Potential
Energy
As implied above, the characteristic component of the free energy of a chemical
substance consists of all forms of available work potential other than the intrinsically
mechanical free energy of concentration. From the standpoint of chemistry, the
characteristic free energy of a substance at a particular temperature consists
only of that associated with what is ordinarily referred to as chemical bond
energy. Bond energies are usually dealt with in terms only of thermal energies
of bond formation or dissociation. This being the case, it is necessary to inquire
as to the general nature of bond energy as a nonthermal actual energy. Since,
other than the extremely weak gravitational force, only Coulomb (electrostatic)
forces and forces deriving therefrom are known to exist between atoms [47],
bond energy as a nonthermal actual energy must consist of these forces and,
since binding energy in respect to a system is defined as the net energy required
to decompose the system into its constituent particles, must correspond to what
is commonly referred to as chemical binding energy.
Since atoms and molecules possess both attractive
and repulsive forces, and since the formation of a kinetically stable chemical
bond at a finite temperature invariably involves the establishment of a balance
between these forces within an energy barrier to the making and breaking of
the bond, chemical binding energy must refer to the energies of both attractive
and repulsive forces. Consider, for example, two kinds of charged atoms A+
and A- which possess binding energy only by virtue of their
net charges. Since unlike charges attract one another, binding energy for a
binding interaction between A+
and A- must necessarily be viewed as being attractive. On
the other hand, since like charges repel, binding energy for an interaction
between A+ and A+,
for example, must necessarily be regarded as being repulsive. Since the attractive
binding interaction would clearly involve a net consumption of binding energy,
the repulsive one must by implication involve a net production of binding energy.
Since both attractive and repulsive binding energy must be forms of nonthermal
actual energy, it appears from this example that the binding energies of molecules
possessing particularly large amounts of bond energy are likely to be of the
repulsive kind. However, as is evident from the fact that the bulk of local
matter occurs naturally as liquids and solids consisting of attractively bound
molecules, most binding interactions between atoms are net attractive. In view
of this and of the large amount of energy required to force like charges into
close proximity to one another, it seems likely that most of the molecules we
ordinarily think of as possessing relatively large amounts of bond energy are
molecules in which the constituent atoms are relatively unattractive to one
another and/or are constrained (bonded) in such a way as to prevent optimal
neutralization of their attractive forces. If we were to consider this invariably
to be the case, we would be ignoring the fact that, by virtue of the existence
of energy barriers to the making and breaking of chemical bonds, it is possible
for kinetically stable bonds to be formed between atoms despite the interaction
being net repulsive.
As is evident from the fact that most chemical
reactions that are thermodynamically favorable in respect to DG° are exothermic, translational kinetic (mechanical)
energy of atoms and molecules can be constrained as bond energy. Uncatalyzed
conversions of translational kinetic energy into bond energy must occur through
collisions of the atoms or molecules with sufficient energy to force the electronic
and other changes (e.g., desolvations) that constitute the energy barrier.
From the fact that bound atoms and molecules tend to dissociate as temperature
is increased, it is evident that the collision energy can be too high as well
as too low.
Since the amount of attractive binding energy
possessed by a substance at constant ambient pressure depends on its temperature
as well as on its chemical composition, it is necessary to distinguish between
changes in attractive binding energy that do and do not constitute changes in
characteristic chemical potential energy. This distinction was in effect made
above by considering thermally produced attractive binding energy to be extramolecular
latent thermal energy. According to the treatment given, the attractive binding
energy that accumulates in an individual substance as its temperature is increased
at constant pressure from absolute zero to the point at which all the molecular
constituents are sufficiently free to be individually mobile is not characteristic
chemical potential energy. That such is the case is evident from the above consideration
of a reversible conversion of ice into liquid water with thermal energy deriving
from nonthermal actual energy used to compress an ideal gas. As was noted, the
free energy of the nonthermal actual energy would remain with the gas rather
than undergo transfer with the thermal energy that would become latent thermal
energy of newly formed liquid.
One can reasonably expect single substances to possess latent thermal energy
consisting of attractive binding energy having the quality of characteristic
chemical potential energy only if the substances are in supercooled states.
Thus, whereas a substance in a superheated state would be out of thermochemical
equilibrium as a result of possessing an excess of translational kinetic energy
relative to attractive binding energy, a substance in a supercooled state would
be out of thermochemical equilibrium as a result of possessing an excess of
attractive binding energy relative to the mechanical energy. That the excess
attractive binding energies of substances in supercooled states actually have
the quality of characteristic chemical potential energy is particularly evident
from studies on single solids that have been mechanically or otherwise reduced
to nano-sized particles at temperatures far below their melting points. Single
solids thus modified are in effect in extremely supercooled states and have
chemical reactivities and other properties, noted above, indicative of the presence
of exceptionally large amounts of characteristic chemical potential energy [48,
49]. For instance, graphite reduced to extremely small
particles by prolonged grinding under inert-gas conditions has been observed
to be sufficiently reactive to ignite spontaneously at room temperature when
exposed to air [50].
Determination of DG° by the Calorimetric Method
Since consumptions and productions of bond free energy under the constant temperature,
constant pressure, and virtually constant volume conditions assumed here are
accompanied by productions and consumptions, respectively, of equivalent amounts
of energy having the quality of thermal energy at the specified constant temperature,
differences in characteristic Gibbs free energy between the reactants and products
of chemical reactions can be determined in closed systems not only by the equilibrium
method, but also by measuring thermal energy changes occurring as a result of
the reactions. However, this 'isothermal' calorimetric method is much more difficult
and less likely to be feasible in that it requires a means of distinguishing
between thermal energy changes due to bond energy changes and irrelevant ones
due to interconversions between the latent and nonlatent thermal energies. An
irrelevant change will occur with any net change in the heat capacity of the
reaction system. Such a change could occur as a result primarily either of a
change in the total intramolecular latent thermal energy of the system or of
a change in the total free energy of concentration (i.e., in the total
number of individually mobile particles in the system). As may be seen by comparing
the reactions A  P +
Q and A2
P +
Q and A2  2A,
a net increase in the total free energy of concentration due to a chemical change
under the conditions assumed here will be accompanied by an increase in a latent
thermal energy that will be indistinguishable from if not identical with the
above-described extramolecular kind.
2A,
a net increase in the total free energy of concentration due to a chemical change
under the conditions assumed here will be accompanied by an increase in a latent
thermal energy that will be indistinguishable from if not identical with the
above-described extramolecular kind.
That free energy of concentration should be
viewed here as elsewhere as being intrinsically mechanical rather than chemical
can be seen by considering a closed Reaction (1) system in which A and P possess
equal amounts of bond energy on a per mol basis and the reaction does not involve
a net change in the system as to latent thermal energy. In such a system, an
interconversion between A and P would not involve a net change in an actual
(First-Law) energy of any kind of either the system or its surroundings. Nevertheless,
an irreversible net conversion would occur and thus be driven with consumption
of free energy if there were a differential in the concentrations of A and P.
Since the system is assumed to be closed, a differential between A and P as
to concentration would be accompanied by differentials as to absolute amount
of both bond energy and translational kinetic energy. Since, for a given differential
in concentration, the magnitude of the driving force for the reaction would
be a function only of temperature, it is evident that, of these two kinds of
actual energy, only the thermal energy would be relevant in respect to the driving
force. However, the fact of the reaction not being accompanied by a change in
actual energy would rule out the thermal energy as well as any other actual
energy of the system as the source of the free energy consumed in driving the
reaction. As will be pointed out below by comparing Reaction (1) systems with
the purely mechanical Figure 1 system, the source of the free energy would be
the nonthermal actual energy used to generate the differential in concentration.
Since only free energy from this source could be present in the closed system,
it would be necessary to attribute the driving force for the reaction simply
to the differential in concentration or 'number density' between A and P. Accordingly,
the reaction would be driven as a result of there being a differential between
the number of A molecules converting spontaneously into P molecules and the
number of P molecules converting spontaneously into A molecules.
Consider now a DG° = 0 reaction which differs from the one
above in that it involves a net change in latent thermal energy and thus a net
change in heat capacity. Since the reaction would not involve a net change in
bond energy and the change in heat capacity and latent thermal energy would occur only in the
system, maintenance of the system at constant temperature would require transmission
of thermal energy between the system and its surroundings. Since for a closed
system such transmission could be driven only by a gradient of temperature,
and since by definition any latent thermal energy produced or consumed at a
particular temperature would have the same quality as nonlatent thermal energy
at that temperature, an interconversion between these energies in such a system
maintained at constant temperature would clearly be irrelevant to the energetics
of the chemical changes that constitute the reaction. In other words, as a result
of the latent and nonlatent thermal energies having different locations and
the same quality at any particular temperature, an interconversion between these
energies in such a system could be driven only by a gradient of temperature,
which, in consequence of the constant-temperature specification, would by definition
be sufficiently small as not to result in a finite consumption of free energy.
From this it is evident that, from the standpoint of theory, a change in latent
thermal energy in any particular reaction system of the sort considered here
could be determined directly from a detectable change in thermal energy only
if the reaction could be conducted reversibly. Consequently, for a reaction
involving a significant change in latent thermal energy, the only way to obtain
a reasonably accurate estimate of DG° by the calorimetric method would be to determine the
difference between the amount of nonlatent (detectable) thermal energy produced
or consumed in the course of the reaction when the reaction is conducted 'completely
irreversibly' (i.e., under conditions of zero work being done on the
surroundings) and the amount produced or consumed when the reaction is conducted
very nearly reversibly (i.e., under conditions in which very nearly all
of the work potential that becomes available in the course of the reaction is
used to do work on the surroundings) (cf. [51, 52]).
For a reaction system maintained at constant
temperature and volume rather than at constant temperature and pressure, the
detectable change in thermal energy occurring under conditions of complete irreversibility
would correspond to the algebraic sum of the changes in the chemical and latent
thermal energies of the system. A change of this sort on a per mol basis is
usually denoted by DE°
and referred to as the difference or change in the total energy of the system.
The detectable change in thermal energy occurring under conditions of near reversibility
would correspond closely to the change in the latent thermal energy of the system
in the opposite direction. Changes of this sort on a per mol basis are denoted
by TDS°. The change in bond energy on a per mol basis would
be given in terms of thermal energy by the difference DE° - TDS° and would correspond closely to the difference between
the reactants and products in respect to characteristic Helmholtz free energy.
Changes in characteristic Gibbs free energy
are reckoned in the same way except that DE° is replaced by DE° + PDV° and referred to as the difference or change in enthalpy,
denoted by DH°. The PDV° component of DH° serves to account for the pressure-volume work that
would be done on the surroundings (atmosphere) by the system or by the surroundings
on the system if the reaction were to result in an increase or decrease, respectively,
in the volume of the system. In consequence of the PDV° component, DG° for a Reaction (1), for example,
could correspond exactly to the difference in bond energy between one mol of
A and one mol of P in their common environment only if DV° for the conversion should happen to be zero. Although
changes in volume ordinarily occur, they are usually small for reactions involving
net changes only in small molecules in solution at constant temperature and
pressure. At normal (atmospheric) pressures the amounts of energy involved are
usually also small and sufficiently so to justify considering them to be negligible
in relation to the change in bond energy.
The above means of estimating and accounting
for differences in the characteristic component of the Gibbs free energy is
in effect a means of doing so in terms of changes in thermal energy of both
the system and its surroundings in terms only of system properties. Thus:
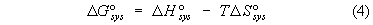
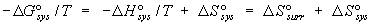
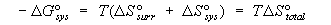
Enthalpy-Entropy
Compensation
The DH° and TDS°
components of the above 'Gibbs standard free energy relation' (Equation 4) are
often referred to as differences or changes in energy and entropy, respectively,
and are often referred to in such a way as to give the impression that the 'energy'
and 'entropy' are different forms of energy that are more or less equally capable
of driving thermodynamic processes under conditions of constant temperature
and pressure. That doing this is not appropriate is evident from the fact that,
since DH° is equivalent to the algebraic sum
of DG° and TDS°
and serves to represent the difference or change in the total energy of the
system, TDS° must represent a difference
or change in an energy of the system that is independent of the difference or
change in thermal energy represented by DG°
and has the quality of thermal energy at the temperature of the system. In other
words, TDS° must serve as indicated above
to represent a difference or change in the kinds of energy referred to as latent
thermal energy. Accordingly, the result DG° = -TDS°
for an isothermal process would mean simply that the change in thermal energy
due to the change in the characteristic Gibbs free energy of the system happens
to be accompanied by an equivalent but oppositely directed change in the latent
thermal energy of the system, and the result DG° = DH° would mean simply that the process does not involve
a net change in the latent thermal energy of the system.
Several problems with current and past interpretations
of the Gibbs standard free energy relation have been pointed out and/or discussed
in recent years by a number of investigators (see, e.g., [53-60]). Some of the problems derive from the fact that reactions
occurring in solution are ordinarily accompanied by a 'solvent reaction' which
is not accounted for in stoichiometric equations and which in some cases appears
to make equivalent contributions of the same sign to DH° and TDS° and thus appears to make little or no contribution
to DG° [53, 61-66]. For reactions occurring in aqueous solution, the energy
of this so-called 'enthalpy-entropy compensation' can be quite large as to amount
and, in such cases where the associated change in DG° is very small, obviously must derive from large conversions
between the latent and nonlatent thermal energies somewhat as in the case of
reversible conversions between the solid and liquid forms of water. In this
regard, it is important to note that, since DH° serves to represent the change in total energy and
thus includes TDS°, enthalpy-entropy compensation in a broad sense must
invariably occur in processes for which TDS° is finite.
Although widely acknowledged to be a phenomenon
that is not well understood, enthalpy-entropy compensation of the solvent-dependent
kind is generally thought to occur as a result of differences between reactants
and products with respect to strength of binding interaction with the solvent
and thus with respect to amount of solvent bound or otherwise immobilized. Such
is consistent with the fact that binding interactions between solutes and solvents
can reasonably be expected to follow the same rules as binding interactions
between solutes. Thus, binding interactions between molecules of a solute and
its solvent to form complexes can be either favorable, with net conversion of
binding energy into thermal energy, or unfavorable, with net conversion of thermal
energy into binding energy. Also, since particles of an undissolved solute idealy
immersed in a solvent would be completely surrounded by the solvent, one could
expect there to be a considerable mechanical (mass-action) driving force (-DGconc) for solvation of the solute from the high solvent
activity alone. Thus, one could expect some solvation of the solute to occur
even if the attractive forces between the solute and solvent molecules should
be very weak. If the attractive forces between molecules of the solute and solvent
should be weaker than those between molecules of the solvent, one could expect
solvation of the solute to be accompanied by net conversion of thermal energy
into attractive binding energy due to hindrance of bond formation between solvent
molecules more or less as proposed by Hildebrand and coworkers [67,
68], resulting in a temperature-sensitive tension in the
solvent at the surfaces of the solute molecules similar to that which occurs
at the interface of the solvent and its vapor. As a result of this tension,
one could expect the existence of a force for minimization of the amount of
space occupied by the solute. Accordingly, surface tension in a liquid results
from molecules at the surface possessing relatively large amounts of attractive
binding energy which in turn results in there being a relatively large attractive
(contractive) force among molecules at the surface that tends to minimize surface
area [69].
Consistent with these expectations, solvations
of nonpolar (hydrophobic) solutes in water tend to be thermodynamically unfavorable
with respect to DG° and the solubilities of such solutes in water tend
to be lower as the surface areas of the molecules are larger (for recent reviews,
see [70-72]). In addition, water has an exceptionally high
surface tension which decreases with increase of temperature [69],
and solvations of nonpolar solutes in water are generally accompanied by a temperature-sensitive
decrease in the amount of space occupied by the solute [73-75].
In contrast to DG°, both DH° and TDS° for solvation tend to be negative, indicating that
the latent thermal energy of the system tends to decrease more than the bond
energy increases. The large negative TDS° associated with the above phenomena, widely referred
to collectively as 'the hydrophobic effect', is usually attributed to a temperature-sensitive
ordering of water molecules at the surfaces of the solute molecules [75-78].
Accordingly, solvation is ordinarily accompanied by an increase in heat capacity
that can be readily explained in terms of reversal of the solvent ordering as
the temperature is increased for the purpose of measuring the heat capacity
[70]. Consistent with the above, 'Hildebrand' interpretation
of the hydrophobic effect, the ordering appears to be limited to a single layer
of water molecules [79].
Contrary to the Hildebrand view, the postulated
ordering of water and the observed increase in bond energy in the hydrophobic
effect have generally been interpreted to mean that there is an increase in
hydrogen bonding, either in number of bonds or in bond strength, and that the
thermodynamics of the ordering process are thus anomalous [72].
If we were to consider the ordering actually to involve an increase in the number
of hydrogen bonds, we could expect it to be accompanied by a decrease in bond
energy rather than by the observed increase and the thermodynamics would in
fact appear to be anomalous, since hydrogen bonding occurs through attractive
(cohesive) forces and therefore can be expected to consume binding free energy.
On the other hand, if we were to consider the ordering to involve only an increase
in hydrogen bond strength and were to interpret 'increase in bond strength'
to mean 'increase in bond energy', we could conceive of the thermodynamics of
the ordering process not being anomalous and of there being no actual disagreement
between the conventional and Hildebrand views. Thus it is conceivable that the
ordering involves only a net increase in the amount of bending and stretching
of hydrogen bonds, in which case there could be an increase in attractive binding
energy without a net decrease in the number of bonds. This possibility is based
on the fact that, by virtue of the existence of energy barriers to the making
and breaking of chemical bonds, the energy of a chemical bond must be enhanced
if the bond is to be broken. In the case of sustained bending or stretching
without net bond breakage, 'enhancement energy' would be retained somewhat as
in the case of the bonds in a stretched rubber band and would consist exclusively
of attractive binding energy. As will be outlined below by considering a solvent
reaction in respect to Reaction (1), the large negative
TDS° and the associated solvent-dependent enthalpy-entropy
compensation in the hydrophobic effect can be explained readily and operationally
in terms of the postulated ordering of solvent molecules simply by taking into
account the fact that the ordering implies immobilization.
In doing this, it shall be assumed (i) that water is the solvent, (ii)
that molecules of the solvent bind less strongly to each other than to A and
P, and (iii) that the attractive binding interaction between the solvent
and P is relatively strong. This being the case, we could expect a net conversion
of A into P to be accompanied by a solvent reaction involving a net conversion
of mobile water into an immobile, bound form and an associated net conversion
of attractive binding energy into thermal energy. In a closed Reaction (1) system,
we could expect this conversion of binding energy into thermal energy to make
a negative contribution to the DG° for the reaction
just as would any other net conversion of binding energy into thermal energy
at constant temperature and pressure. Therefore, we could expect the contributions
to DH° and TDS° to differ accordingly. How, then, can a difference
between the reactant and product with regard to solvent immobilization account
for solvent-dependent enthalpy-entropy compensation? As surmised by Ives and
Marsden [62], this question can be answered by taking into
account changes in a specific extensive (size) variable of closed reaction systems.
Enthalpy-entropy compensation of the above sort can be adequately explained
by invoking the above-noted prediction of the equipartition principle that,
at any particular temperature, the individually mobile particles of a gas will
possess on average the same amount of translational kinetic energy regardless
of the nature of the particles. That this prediction is generally considered
to be correct and applicable to substances in ideal solution is evident from
the fact that it is implicit in the concentration term of the reaction isotherm
(Equation 2) through the assumption that, at any particular
temperature, only the relative concentrations of A and P as particles matters
in regard to the difference in free energy of concentration. According to this
assumption, differences in the characteristic features of A and P, such as size
(mass), net charge, amount of bond energy, amount of bound solvent, and amount
of intramolecular latent thermal energy, are of no consequence whatever as regards
the contributions of A and P to free energy of concentration. In regard to particle
size, it may be noted that Einstein [80] employed the above
assumption in his very successful treatment of Brownian movements of particles
of sufficient size to be seen by light microscopy. This and the fact that one
could reasonably expect to be capable of demonstrating the equilibrium value
of RT ln( p/a) for a Reaction (1) as a difference in mechanical
(osmotic) work potential equivalent to the DG° for the reaction imply (i) that differences
in free energy of concentration are differences in the density of translational
kinetic energy and (ii) that, at any particular temperature, individually
mobile particles of A and P would possess on average over time equivalent amounts
of translational kinetic energy regardless of their characteristic features.
This being the case, one could reasonably expect equipartition of the translational
kinetic energy of a closed reaction system among all the particles of the system,
including, of course, those of the solvent.
Accordingly, if P of a closed Reaction (1)
system were to immobilize more water than does A, one could expect a net conversion
of A into P to be accompanied by (i) a decrease in the number of particles
in the system, (ii) an equipartitioning of the total translational kinetic
energy of the system among the smaller number of particles, (iii) an
augmentation of the average translational kinetic energy of the particles, (iv)
an elevation of the temperature of the system, and (v) a transmission
of thermal energy from the system to the surroundings. Since the decrease in
number of particles would occur regardless of the driving force for the conversion
of A into P and thus regardless of whether the reaction is conducted reversibly
or irreversibly, it would make a negative contribution to both DH° and TDS°. Of course if A were to immobilize more water than
does P, the sign of the contribution would be positive rather than negative.
In either case, the contribution could be regarded as being due to a change
in free energy of concentration that is not accounted for in the stoichiometric
equation for the reaction.
Since the binding of water to solute and of
water to water can be expected to vary with temperature, one could expect the
magnitude of the contribution to be temperature dependent. For reactions involving
net changes in large biological molecules, in which cases changes in latent
thermal energy on a per mol basis tend to be particularly large, this dependence
is particularly noticeable. A question currently of interest in this regard
is why applications of the integrated form of the van't Hoff equation:
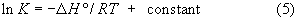
to reactions of the above sort do not yield
correct values for DH° despite yielding linear plots of ln K vs. 1/T
[54-60]. This apparent discrepancy can be resolved by noting
that, since ln K is also equivalent
to -DG°/RT, the integration constant of Equation (5)
must be equivalent to DS°/R if the equation is to be consistent with
Equation (4), and that, since the validity of Equation
(4) is beyond dispute, the right-hand side of Equation (5) must therefore be
equivalent to -DG°/RT. This being the case, linearity of plots
of ln K vs. 1/T
means nothing more than that ln K
is directly proportional to -DG°/T, the observed proportionality constant being
an estimate of 1/R, and that DG° is a linear function of T.
Role of Free Energy of Concentration in
Free Energy Transfer and Conservation
As was noted above, the nature of free energy of concentration is fundamentally
the same for a system in which only chemical work is possible as for an ideal
gas system in which only mechanical work is possible. Accordingly, the concentration
term of the reaction isotherm ignores completely the presence of the solvent.
Thus, RT ln( p/a)
of Equation (2) would be applicable to a closed Reaction (1) system even if A and P were gases in an ideal
gas-phase reaction. This being the case, we can apply to a closed Reaction (1)
system virtually everything said above concerning the energy and entropy changes
of an ideal gas associated with compression and expansion of the gas between
two states at the same temperature simply by ignoring the means by which the
two states were assumed to be achieved and considering a and p
to be the two states. The fact that the volume would be essentially constant
for the two states in the closed Reaction (1) case would not matter because
the difference in free energy of concentration between them on a per mol basis
could be determined from knowledge of the relative values of a and p.
The fact that A and P would share the same space also would not matter because
A and P would be virtually independent of one another in the ideal case and
thus may be thought of as being individual systems within an overall Reaction
(1) system.
The individual systems of a closed Reaction (1) system may be thought of as
being pitted against one another in respect to compression and expansion. For
instance, if a and p were equal and A possessed RT ln10 more bond energy than P on a per
mol basis, the A system, through 'expansion', would be capable of 'compressing'
the P system to the extent that p exceeds a by a factor of ten,
at which point (i) the reaction would be at equilibrium, (ii)
the amount of bond energy in the individual systems would be the same on an
absolute amount basis, and (iii) the A and P systems would differ as
to free energy of concentration on a per mol basis by RT ln10.
Since at equilibrium the absolute amount of
bond energy would be the same for the A and P systems, the difference in free
energy of concentration, although generated as a result of there having been
a difference in bond energy on an absolute amount basis, obviously would not
be associated with such a difference at equilibrium. Therefore, since any consumption
of bond free energy in the course of the reaction going to equilibrium would
be accompanied by the production of an equivalent amount of thermal energy,
and since all of this thermal energy could depart the system as a result of
the temperature being maintained constant if the conversion of A into P should
happen not to result in an increase in the latent thermal energy of the system,
the free energy of concentration generated in the P system, like that which
one could expect to be generated in an ideal gas upon its isothermal compression,
could appear to be free of the actual energy that undergoes transformation in
the course of its generation.
We turn now to the question of how the difference
in free energy of concentration could be utilized to do chemical work (i.e.,
to convert ambient thermal energy into chemical bond energy). As noted above,
Reaction (1) as presented is assumed to be linked to others through A being
a product of a preceding reaction and through P being a reactant of a succeeding
one in a stationary-state, biological-type system. To rule out the possibility
of differences in free energy of concentration being used to do mechanical (osmotic)
work in addition to chemical work, the individual reactions of this system shall
be assumed to be located in the same compartment. If Reaction (1) were thermodynamically
favorable in respect to DG°, a difference in free energy of concentration of the
above sort could be generated and conserved as bond free energy through the
differential between a and p either pulling preceding reactions
or pushing succeeding ones that are thermodynamically unfavorable in respect
to DG° and thus require thermal energy to proceed in the
same direction as Reaction (1). If the individual reactions were coupled only
by freely diffusible intermediates, transmissions of actual energy between the
reactions would occur only through transmissions of thermal energy. Of course
the role of the differential in free energy of concentration in conserving the
excess bond free energy of A relative to P on a per mol basis would be to increase
the number of reactant molecules converting spontaneously into product molecules
relative to the number of product molecules converting spontaneously into reactant
molecules in the unfavorable reactions. If the stationary-state ratio of p
to a were infinitesimally smaller than the equilibrium constant of Reaction
(1), all of the reactions would be operating reversibly in the thermodynamic
sense and all of the bond free energy would be conserved as bond energy.
Although the biological system as one in which
only chemical work is possible is similar to the purely mechanical (Figure
1) system in that it employs a difference in density of translational kinetic
energy to mediate the transfer of free energy from one repository to another,
it differs markedly from the purely mechanical system in that it employs the
particle-number-density (concentration) aspect rather than the mechanical-energy-density
aspect of the translational energy density. Owing to the need for thermal energy
transfer between the system and surroundings at a low temperature differential
to conserve free energy in the purely mechanical case and the lack of such need
to conserve free energy in the biological case, the two systems also differ
greatly as to the amount of time that would be required for efficient transfer
of the actual energy. Thus, in the biological case the reactions yielding and
requiring thermal energy could occur simultaneously in close proximity to one
another at the microscopic level, a consequence of which would be that the requisite
thermal energy transfers could occur rapidly while the temperature of the system
is essentially constant. Thermal energy transfer between the biological system
and its surroundings would be needed only to rid the system of the thermal energy
produced as a result of the need for expenditure of bond free energy to maintain
an appropriate net rate of reaction. If the system should require thermal energy
to maintain its temperature, this expenditure of free energy would also serve
to meet this need.
Appendix
According to the main body of the text, by
considering irreversibility in respect to an adiabatic cycle of compression
and expansion of an ideal gas, one can readily see that any irreversibility
in a thermodynamic work process would be reflected only in the characteristic
entropy and would result in the consumption of an amount of characteristic free
energy equivalent to an amount of ambient thermal energy produced. That these
observations are correct may be seen from the following considerations in reference
to an adiabatic version of the Figure 1 model of the main
body of the text.
If one mol of an ideal gas in the fully expanded
state V1 at a temperature T1 of the reservoir
were compressed to a volume V2 adiabatically, the temperature
of the gas would increase by an amount depending on the heat capacity and on
the amount of energy DE
imparted to the gas. This amount of energy would be equivalent to the amount
of work W done and thus would depend on the rate of the compression.
Regardless of the rate, W and DE would be equivalent to CV DT. Since S, T, and V are state
functions and CV would be a constant, the expression for the
net change in entropy
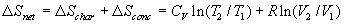
in terms of these parameters would be applicable regardless of the rate of
the compression. If the gas were compressed reversibly, DSchar
would be equivalent to -DSconc
and T2 could be determined
from the relationship 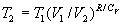 .
If the gas were compressed at a finite
rate, T2 would be relatively high and DSne t
for the compression would be finite and positive as a result of the increase
in characteristic entropy exceeding the decrease in entropy of concentration.
In this 'irreversible' case, T2 could be determined only by
experimental means. Although calculation of DSne t
could be easily achieved if T2 for the irreversible compression
were known, doing so would not be helpful in respect to precise quantification
of the amount of free energy consumed. However, since the work potential of
the gas would increase by an amount equivalent to the amount of work done regardless
of the rate of the compression, doing so could serve to indicate in a semiquantitative
fashion that work potential has been rendered unavailable. As indicated below,
if T2 were known, the precise amount rendered unavailable
could be determined theoretically by considering the gas to undergo reversible
expansion to its original volume.
.
If the gas were compressed at a finite
rate, T2 would be relatively high and DSne t
for the compression would be finite and positive as a result of the increase
in characteristic entropy exceeding the decrease in entropy of concentration.
In this 'irreversible' case, T2 could be determined only by
experimental means. Although calculation of DSne t
could be easily achieved if T2 for the irreversible compression
were known, doing so would not be helpful in respect to precise quantification
of the amount of free energy consumed. However, since the work potential of
the gas would increase by an amount equivalent to the amount of work done regardless
of the rate of the compression, doing so could serve to indicate in a semiquantitative
fashion that work potential has been rendered unavailable. As indicated below,
if T2 were known, the precise amount rendered unavailable
could be determined theoretically by considering the gas to undergo reversible
expansion to its original volume.
In contrast to what would be the case for an ideal gas compressed irreversibly
between two states at the same temperature, reversible expansion of the adiabatically
compressed gas would result in a fraction of the thermal energy produced as
a result of the gas being compressed at a finite rate being recovered as work,
the fraction being that indicated by the efficiency of a Carnot engine operating
reversibly between T2
and the lower temperature that would exist upon return of the gas to its original
volume V1. Since DSchar
for the expansion would be equivalent to -DSconc,
this lower temperature would be equivalent to 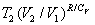 and would of course
be higher than the temperature T1
of the reservoir by an amount depending on how fast the gas was compressed.
In addition to the difference in temperature, there would remain differences
in the pressure, thermal energy, and characteristic entropy, the difference
in characteristic entropy being equivalent to that existing at the end of the
compression phase. Elimination of these differences would require removal of
the adiabatic constraint so that an amount of thermal energy CV DT
could undergo transmission to the reservoir, CV DT
in this case being equivalent to the amount of characteristic free energy consumed
as a result of the gas being compressed at a finite rate. Since the amount of
the gas was specified to be one mol, this amount would be equivalent to
and would of course
be higher than the temperature T1
of the reservoir by an amount depending on how fast the gas was compressed.
In addition to the difference in temperature, there would remain differences
in the pressure, thermal energy, and characteristic entropy, the difference
in characteristic entropy being equivalent to that existing at the end of the
compression phase. Elimination of these differences would require removal of
the adiabatic constraint so that an amount of thermal energy CV DT
could undergo transmission to the reservoir, CV DT
in this case being equivalent to the amount of characteristic free energy consumed
as a result of the gas being compressed at a finite rate. Since the amount of
the gas was specified to be one mol, this amount would be equivalent to 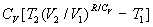 .
The value thus obtained divided by the temperature of the reservoir would be
the net increase in entropy of the reservoir and for the overall process. As
in any cyclic process, the net changes in free energy and entropy would be changes
only in the characteristic kinds. Since the increase in entropy for the overall
process would differ from that incurred by the gas in the compression phase
and could be determined quantitatively only through determination of the amount
of thermal energy transmitted to the surroundings, quantification of the net
change in entropy for the cyclic process would in effect require prior quantification
of the net change in free energy.
.
The value thus obtained divided by the temperature of the reservoir would be
the net increase in entropy of the reservoir and for the overall process. As
in any cyclic process, the net changes in free energy and entropy would be changes
only in the characteristic kinds. Since the increase in entropy for the overall
process would differ from that incurred by the gas in the compression phase
and could be determined quantitatively only through determination of the amount
of thermal energy transmitted to the surroundings, quantification of the net
change in entropy for the cyclic process would in effect require prior quantification
of the net change in free energy.
Acknowledgement: This work was supported in part by the Stoner
Research Fund of the Ohio State University Development Fund.
References
- van't Hoff, J. H. The Role of Osmotic Pressure in the Analogy between
Solutions and Gases. Alembic Club Reprints 1929, 19,
5-42. [Translation of: Z. Physik. Chem. 1887, 1, 481-508.]
- Zemansky, M. W.; Dittman, R. H. Heat and Thermodynamics;
6th ed.; McGraw-Hill: New York, 1981; pp.160-161.
- Stoner, C. D. An Investigation
of the Relationships between Rate and Driving Force in Simple Uncatalysed and
Enzyme-Catalysed Reactions with Applications of the Findings to Chemiosmotic
Reactions. Biochem. J. 1992, 283, 541-552.
- Lewis, G. N.; Randall, M.; Pitzer, K. S.; Brewer, L. Thermodynamics;
2nd ed.; McGraw-Hill: New York, 1961; p. 13.
- Clausius, R. The Mechanical Theory of Heat; Browne, W. R.,
Translator; Macmillan: London, 1879; pp.69-109.
- Glasstone, S. Thermodynamics for Chemists; Van Nostrand: New York,
1947; pp. 51-60.
- Gurney, R. A. Ionic Processes in Solution; McGraw-Hill:
New York,1953; pp. 80-112.
- Carnot, S. Reflexions
on the Motive Power of Fire; Fox, R., Translator; Lilian-Barber: New York,1986.
- d'Abro, A. The Rise of the New Physics; Dover: New York, 1951; Chaps. 22 and 40.
- Bent, H. A. The Second
Law; Oxford: New York, 1965; pp. 32-133.
- Born, M. Atomic Physics; Dover: New York, 1989; Chaps.
7 and 9.
- Kauzmann, W. Kinetic Theory of Gases; Benjamin:
New York, 1966; pp. 91-130.
- Hinshelwood, C. N. The Structure of Physical Chemistry; Oxford: London,
1965; pp. 1-24.
- Denbigh, K. G. The
Principles of Chemical Equilibrium; 4th ed.; Cambridge University
Press: London, 1981; pp. 196-214.
- Eisenberg, D.; Kauzmann, W. The Structure and Properties
of Water; Oxford: New York, 1969.
- Frank, H. S.; Wen, W.-Y. Structural Aspects of Ion-Solvent
Interaction in Aqueous Solutions: A Suggested Picture of Water Structure.
Discuss. Faraday Soc. 1957, 24, 133-140.
- Némethy, G.; Scheraga, H. A. Structure of
Water and Hydrophobic Bonding in Proteins. I. A Model of the Thermodynamic Properties of Liquid Water. J. Chem. Phys. 1962, 36, 3382-3400.
- Liu, K.; Cruzan, J. D.; Saykally, R. J.
Water clusters. Science 1996, 271, 929-933.
- McQuarrie, D. A. Statistical
Mechanics; Harper Collins: New York, 1976; pp. 194-217.
- Kaganov, K. I.; Lifshits,
I. M. Quasiparticles; Kissen, V., Translator; Mir: Moscow, 1979.
- Barnes, H. T.; Vipond, W. The Sublimation of Ice. Phys. Rev. 1909, 28, 453.
- Barnes, H. T. Ice Engineering; Renouf: Montreal, 1928; pp. 31-33.
- Benedicks, C. Über die
Herleitung von Plancks Energieverteilungsgesetz aus Agglomerationsannahme; einfache
Beziehung zwischen Härte und Schwingungszahl. Ann. Phys. 1913, 42, 133-162.
- Compton, A. H. The Variation
of the Specific Heat of Solids with Temperature. Phys. Rev. 1915, 6,
377-389.
- Debye, P. On the Theory
of Specific Heats. In The Collected Papers of Peter J. W. Debye; Interscience: New York, 1954; pp.650-696.
- Compton, A. H. A Physical
Study of the Thermal Conductivity of Solids. Phys. Rev. 1916, 7, 341-348.
- Valentiner, S. Die Grundlagen der Quantentheorie in elementarer
Darstellung; 3. erw. Aufl.; Vieweg und Sohn: Braunschweig, 1920; p. 19.
- Mehra, J.; Rechenberg, H. The Historical Development of Quantum Theory; Springer: New York,1982; Vol. 1, Part 1, p. 151.
- Benedicks, C. Metallographic
Researches; McGraw-Hill: New York,1926; Chap. 1.
- Klein, M. J. Einstein
and the Wave-Particle Duality. The Natural Philosopher 1964, 3, 3-49.
- de Broglie, L. Sur
les Interférences et la Théorie des Quanta de Lumière. Comptes Rendus Acad.
Sci. (Paris) 1922, 175, 811-813.
- de Broglie, L. Black
Radiation and Light Quanta. In Selected Papers on Wave Mechanics; de Broglie, L.; Brillouin, L., Eds.; Blackie and Son: London, 1928; pp. 1-7.
- MacKinnon, E. De Broglie's thesis: A critical
retrospective. Am. J. Phys. 1976, 44, 1047-1055.
- Tonomura, A.; Endo, T.; Matsuda,
T.; Kawasaki, T. Demonstration of Single-Electron Buildup of an Interference Pattern. Am. J. Phys. 1989, 57, 117-120.
- Zeilinger, A.; Gähler, R.; Shull,
C. G.; Treimer, W.; Mampe, W. Single- and Double-Slit Diffraction
of Neutrons. Revs. Mod. Phys. 1988, 60, 1067-1073.
- Gleiter, H. Nanocrystalline Materials. Prog. Mater. Sci. 1989, 33, 223-315.
- Greer, A. L. Thermodynamics of Nanostructured Materials. In Mechanical Properties and Deformation Behavior of Materials Having Ultra-Fine Microstructures; Nastasi, M.; Parkin, D.
M.; Gleiter, H., Eds.; Kluwer: Dordrecht and Boston,1993; pp. 53-77.
- Alivisatos, A. P. Perspectives
on the Physical Chemistry of Semiconductor Nanocrystals. J. Phys. Chem. 1996, 100, 13226-13239.
- Fecht, H. J. Formation of Nanostructures by Mechanical Attrition. In Nanomaterials: Synthesis,
Properties and Applications; Edelstein, A. S.; Cammarata, R. C., Eds.; Institute of Physics Publishing: Bristol and Philidelphia, 1996; pp. 89-110.
- Borel, J.-P. Thermodynamic Size Effect and the Structure of Metallic Clusters. Surf. Sci. 1981, 106, 1-9.
- Vergara, O.; Heitkamp, D.; Löhneysen, H. v. Specific Heat of Small Vanadium Particles in the Normal and Superconducting State. J. Phys. Chem. Solids 1984, 45, 251-258.
- Jura, G.; Pitzer, K. S. The Specific Heat of Small Particles at Low Temperatures. J. Am. Chem. Soc. 1952, 74, 6030-6032.
- Simon, F. The Third Law of Thermodynamics; An Historical Survey. Yearbook of the Physical Society
(Great Britain) 1956, 1956, 1-22.
- Zemansky, M. W. Temperatures
Very Low and Very High; Dover: New York, 1964; p. 76.
- Pitzer, K. S. Thermodynamics;
3rd ed.; McGraw-Hill: New York, 1995; pp. 82-97.
- Boggs, J. E. The Logarithm
of "Ten Apples". J. Chem. Educ. 1958, 35, 30-31.
- Moore, W. J. Physical
Chemistry; 4th ed.; Prentice-Hall: New York, 1972; pp. 913-915.
- Lin, I. J.; Nadiv, S. Review of the Phase Transformation and Synthesis of Inorganic Solids Obtained by Mechanical Treatment (Mechanochemical Reactions). Mater. Sci. Eng. 1979, 39, 193-209.
- Hermann, H.; Schubert, T.; Gruner,
W.; Mattern, N. Structure and Chemical Reactivity of Ball-Milled
Graphite. Nanostruc. Mater. 1997, 8, 215-229.
- Kosmac, T.; Courtney, T. H. Milling and Mechanical Alloying of Inorganic Nonmetallics. J. Mater. Res. 1992, 7, 1519-1525.
- Maron, S. H.; Lando, J. B. Fundamentals of Physical Chemistry; Macmillan: New York, 1974; pp. 329-331.
- Klotz, I. M.; Rosenberg,
R. M. Chemical Thermodynamics; 5th ed.; Wiley: New
York, 1994; pp. 129-130.
- Lumry, R. On the Interpretation
of Data from Isothermal Processes. Methods Enzymol. 1995, 259, 628-720.
- Weber, G. Van't Hoff Revisited: Enthalpy of Association of Protein Subunits. J. Phys. Chem. 1995, 99,
1052-1059.
- Ragone, R.; Colonna, G. Reliability of van't Hoff Plots. J. Phys. Chem. 1995, 99, 13050.
- Naghibi, H.; Tamura, A.;
Sturtevant, J. M. Significant Discrepancies between van't Hoff and Calorimetric Enthalpies. Proc. Natl. Acad. Sci. U.S.A. 1995, 92, 5597-5599.
- Weber, G. Persistent Confusion of Total Entropy and Chemical System Entropy in Chemical Thermodynamics. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 7452-7453.
- Ross, J. Comments on the
Article "Persistent Confusion of Total Entropy and Chemical System Entropy in
Chemical Thermodynamics" [Proc. Natl. Acad. Sci. U.S.A. 1996, 93,
7452-7453]. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 14314.
- Holtzer, A. Persistent
Confusion on the van't Hoff Equation. Biopolymers 1997, 42, 499-503.
- Liu, Y.; Sturtevant, J. M. Significant Discrepancies between van't Hoff and Calorimetric Enthalpies. III. Biophys. Chem. 1997, 64, 121-126.
- Leffler, J. E.; Grunwald,
E. Rates and Equilibria of Organic Reactions; Wiley:
New York, 1963; pp. 40-56.
- Ives, D. J. G.; Marsden,
P. D. The Ionization Functions of Di-Isopropylcyanoacetic Acid in Relation
to Hydration Equilibria and the Compensation Law. J. Chem. Soc. 1965, 1965,
649-676.
- Lumry, R.; Rajender, S.
Enthalpy-Entropy Compensation Phenomena in Water Solutions of Proteins and Small Molecules: A Ubiquitous Property of Water. Biopolymers 1970, 9, 1125-1227.
- Gilli, P.; Ferretti, V.; Gilli,
G.; Borea, P. A. Enthalpy-Entropy Compensation in Drug-Receptor Binding.
J. Phys. Chem. 1994, 98, 1515-1518.
- Dunitz, J. D. Win Some,
Lose Some: Enthalpy-Entropy Compensation in Weak Intermolecular Interactions.
Chem. Biol. 1995, 2, 709-712.
- Grunwald, E. Thermodynamics
of Molecular Species; Wiley: New York, 1997; pp. 120-136.
- Miller, K. W.; Hildebrand,
J. H. Solutions of Inert Gases in Water. J. Am. Chem. Soc. 1968, 90,
3001-3004.
- Hildebrand, J. H.; Prausnitz, J. M.; Scott, R. L. Regular and Related Solutions;
Van Nostrand Reinhold: New York, 1970; pp.137-141.
- Davies, J. T.; Rideal, E. K. Interfacial Phenomena; Academic Press: New York, 1963; pp. 1-54.
- Privalov, P. L.; Gill, S. J. The Hydrophobic Effect: A Reappraisal. Pure Appl. Chem. 1989, 61,
1097-1104.
- Muller, N. Search for a Realistic View of Hydrophobic
Effects. Acc. Chem. Res. 1990, 23, 23-28.
- Blokzijl, W.; Engberts, J.
B. F. N. Hydrophobic Effects. Opinions and Facts. Angew. Chem. Int.
Ed. Engl. 1993, 32, 1545-1579.
- Masterton, W. L. Partial
Molal Volumes of Hydrocarbons in Water Solution. J. Chem. Phys. 1954, 22,
1830-1833.
- Edsall, J. T.; Wyman, J. Biophysical
Chemistry; Academic Press: New York, 1958; pp. 27-46.
- Kauzmann, W. Some Factors
in the Interpretation of Protein Denaturation. Adv. Protein Chem. 1959, 14,
1-63.
- Frank, H. S.; Evans, M. W. Free Volume and
Entropy in Condensed Systems. III. Entropy in Binary Liquid Mixtures; Partial
Molal Entropy in Dilute Solutions; Structure and Thermodynamics in Aqueous Electrolytes.
J. Chem. Phys. 1945, 13, 507-532.
- Némethy, G.; Scheraga, H. A. Structure of
Water and Hydrophobic Bonding in Proteins. II. Model for the Thermodynamic Properties of Aqueous Solutions of Hydrocarbons. J. Chem. Phys. 1962, 36, 3401-3417.
- Edsall, J. T.; Gutfreund, H. Biothermodynamics;
Wiley: New York, 1983.
- Gill, S. J.; Dec, S. F.; Olofsson,
G.; Wadsö, I. Anomalous Heat Capacity of Hydrophobic Solvation. J. Phys. Chem. 1985, 89, 3758-3761.
- Einstein, A. Investigations
on the Theory of the Brownian Movement; Cowper, A. D., Translator; Dover:
New York, 1956.
© 2000 by the author. Reproduction of this article for noncommercial
purposes is permitted.
