Ciba-Geigy Limited, Pharmaceuticals Division, CH-4002 Basel, Switzerland
*Present Address: Molecular Diversity Preservation International (MDPI), Saengergasse 25, CH-4054 Basel, Switzerland. Tel. +41 79 322 3379, Fax +41 61 302 8918 ([email protected])
Received: 15 January 1997 / Published: 15 April 1997

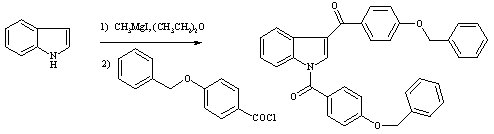
The general part of the experimental section [1] has been presented elsewhere. A stirred solution of indole (3.039g, 25.94mmol) in diethyl ether (40ml) was treated dropwise with methylmagnesium iodide solution [freshly prepared by addition of a solution of methyl iodide (1.65ml, 25.9mmol) in diethyl ether (10ml) to Mg (0.6307g, 25.94mmol) in diethyl ether (5ml)] at 0deg.C and stirred for 1.5 hr after removal of the ice/water bath. The final temperature was 22deg.C. A solution of 4-benzyloxybenzoyl chloride (6.40g, 25.94mmol) in 50ml THF was quickly added without delay. Stirring was then continued at 22deg.C for 1hr. The reaction mixture was poured into a mixture of water (250ml), HCl (1N, 22ml) and ice (50g). NaOH (2N, ca. 10ml) was added to give pH 7. Extraction with ethyl acetate (10 times 80ml) was followed by evaporation and filtration to yield 3.515 g (50.4%) of the title compound, a white powder.
M.p. 180-181deg.C.
TLC (Hexane/EtOAc 1:1): Rf 0.67.
1H-NMR (CDCl3): 8.25 (m, 2H); 7.75-7.90 (m, 5H); 7.42 (m, 12H); 7.08 (m, 4H); 5.17 (d, 4H).
Anal. calc. for C36H27NO4 (537.61): C 80.43, H 5.06, N 2.61; found: C 80.04, H 5.18, N 2.57.
Acknowledgments: The author would like to thank Dr. Vittorio Rasetti for his support.
References and Notes
1. Lin, S. -K.; Rasetti, V. Synthesis of benzospiro[5,6]undecane derivatives as inhibitors of steroid 5-a-reductase. Helv. Chim. Acta. 1995, 78, 857-865.
Sample Availability: Available from MDPI, 0.1745g, MDPI 9940.
© 1997 MDPI. All rights reserved