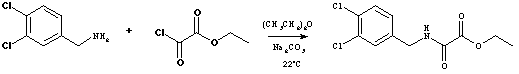
Shu-Kun Lin*
Ciba-Geigy Limited, Pharmaceuticals Division, CH-4002 Basel, Switzerland
*Present Address: Molecular Diversity Preservation International (MDPI), Saengergasse 25, CH-4054 Basel, Switzerland. Tel. +41 79 322 3379, Fax +41 61 302 8918 ([email protected])
Received: 15 January 1997 / Published: 15 April 1997
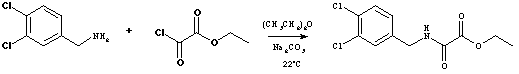
The general part of the experimental section [1] and the purposes of this synthesis [2] have been presented elsewhere. A solution of K2CO3 (19.9 g, 144 mmol) in water (50ml) was added to a suspension of 3,4-dichlorobenzylamonium chloride (8.29 g, 39.01 mmol) in diethyl ether (250 ml) and stirred for 3 min to give the free amine. This mixture was cooled down to 0deg.C and ethyl oxalyl chloride (5.86 g, 42.9 mmol) was added dropwise over a period of 10 min at 0deg.C. After stirring at r.t. for 2 h, the organic phase was washed with HCl (0.001N, 200ml), water (2x200ml) and dried (Na2SO4). Slow evaporation overnight produced 9.84 g (91.3%) of the title compound as large crystals.
M.p. 71-72deg.C.
TLC (Hexane/EtOAc 2:1): Rf 0.13.
IR (CH2Cl2): 3412, 3058, 2941, 2902, 1760 (COOEt), 1706 (C=O), 1523, 1471, 1400.
1H-NMR (CDCl3): 7.41 (bs, 1H, NH); 7.40 (m, 2H); 7.15 (m, 1H); 4.46 (d, 4H, CH2); 4.35 (q, 2H), 1.40(t, 3H).
(+)-FAB-MS: 276 ([M+H]+).
Anal. calc. for C11H11Cl2NO3 (276.12): C 47.85, H 4.02, N 5.07, Cl 25.68; found: C 47.99, H 4.03, N 5.13, Cl 25.62.
Acknowledgments: The author would like to thank Dr. Vittorio Rasetti for his support.
References and Notes
1. Lin, S. -K.; Rasetti, V. Synthesis of benzospiro[5,6]undecane derivatives as inhibitors of steroid 5-a-reductase. Helv. Chim. Acta. 1995, 78, 857-865.
2. Lin, S. -K. A facile synthesis of quinoxaline-2,3-diones as NMDA receptor antagonists. Molecules 1996, 1, 37-40.
Sample Availability: Not available.
© 1997 MDPI. All rights reserved