Institute of Organic Chemistry, University of Lausanne, BCH, CH-1015
Lausanne-Dorigny, Switzerland.
Fax: ++ 41 21 692 3955 ([email protected])
Received: 1 February 1997 / Published: 15 April 1997

Scheme
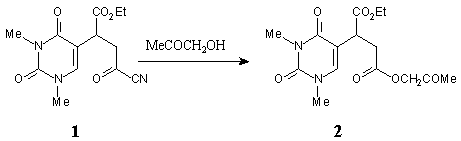
The diester 2 was prepared by the addition of 2-hydroxyacetone to 1 according to the reported procedure [1].
2-Hydroxyacetone (0.2 ml, 2.9 mmol) was added to a solution of 1 (293 mg, 1 mmol) in CH2Cl2 (5 ml). The mixture was left at r.t. for 2 h and and filtered through a pad of silica gel (EtOAc) afforded the title compound 2, a colourless oil: 320 mg (94 %).
IR (neat): 3070m, 1725vs, 1700vs, 1660vs, 1640vs, 1483s, 1460s, 1435s, 1375s, 1350s, 1275s, 1250s, 1190s, 1155s, 1090s, 1020s, 780s, 755s.
1H-NMR (CDCl3): 7.30 (s, 1H, H-6'); 5.66 (s, 2H, CO2CH2COCH3); 4.16 (q, J = 7.1, 2H, CO2CH2Me), 3.88 (dd, J = 8.2, 5.7, H-2); 3.41 (s, Me-1'); 3.32 (s, Me-3'); 3.18 (dd, J = 16.8, 5.7, 1H, H-3); 2.92 (dd, J = 16.8, 8.2, 1H, H-3); 2.13 (s, 3H, CO2CH2COCH3); 1.23 (t, J = 7.1, CO2CH2CH3).
13C-NMR (CDCl3): 207.5 (CO2CH2COCH3), 173.4 (CO2CH2COCH3), 171.3 (CO2CH2Me), 162.0 (C-4'), 151.0 (C-2'), 141.7 (C-6'), 109.7 (C-5'), 67.9 (CO2CH2COCH3), 60.8 (CO2CH2Me), 39.5 (C-2), 36.5 (Me-3'), 34.2 (C-3), 27.3 (Me-1'), 24.7 (CO2CH2COCH3), (CO2C(CH3)3), 13.4 (CO2CH2CH3).
EI-MS: 340 (M+, 4), 295 (4), 294 (10), 267 (5), 266 (13), 239 (2), 238 (8), 237 (6), 194 (12), 193 (100), 169 (2), 167 (5), 166 (13), 165 (11), 110 (2), 81 (15), 80 (8), 69 (2), 68 (4), 56 (2).
Acknowledgment: We thank the Swiss National Foundation for financial support.
References
1. Zhuo, J.-C.; Wyler, H. Helv. Chim. Acta 1993, 76, 1916.
Sample Availability: Available from MDPI, 0.3g, MDPI 10059.
© 1997 MDPI. All rights reserved