Radek Marek
Department of Organic Chemistry, Faculty of Science, Masaryk University, Kotlárská 2, CZ-611 37 Brno, Czech Republic. Tel. 00420-5-41129383, Fax. 00420-5-41211214 ([email protected]). http://www.chemi.muni.cz/nmr/radek/radek.html
Received: 14 May 1997 / Published: 20 May 1997
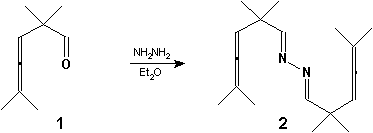
The homoallenylaldehyde 1 was prepared according to the literature [1,2]. Half an equivalent of hydrazine monohydrate [3] was added dropwise to a solution of 1 with a catalytic amount of p-TSA in diethylether [4]. The mixture was stirred at r.t. for 2 h, evaporated under reduced pressure to remove the solvent. The product was purified by column chromatography on silica gel using CH2Cl2 to give the title compound (2). Liquid, 38% yield.
nD20 = 1.4910.
TLC (CHCl3): Rf = 0.52.
MS (EI): 257 ([M-CH3]+), 163, 148, 136, 122, 79, 67
IR (neat): 740 (s), 810 (s), 900 (w), 1010 (w), 1100 (w), 1195 (w), 1255 (m), 1370 (w), 1460 (w), 1630 (C=N), 1950 (C=C=C), 2860 (w), 2920 (w), 2960 (s).
1H NMR (acetone-d6): 1.14 (12H, s, (CH3)2C), 1.63 (12H, d, (CH3)2C=C=C, 5J=2.8 Hz), 4.89-4.97 (2H, sept, HC=C=C, 5J=2.8 Hz), 7.50 (2H, s, HC=N).
13C NMR (acetone-d6): 20.66 (C6, C7), 25.51 (C8, C9), 38.93 (C2), 96.38 (C3), 98.02 (C5), 168.31 (C1), 200.61 (C4).
Acknowledgment: The author would like to thank Mgr. Irena Stastná for her support.
References
1. Black, D.K.; Landor S.R. J. Chem. Soc. 1965, 6784.
2. Marek, R.; Potácek, M.; Marek, J.; De Groot, A.; Dommisse, R. Monatsh. Chem. 1995, 126, 1151.
3. Marek, R.; Potácek, M.; Sapík, M. Tetrahedron Lett. 1995, 36, 8101.
4. Potácek, M.; Marek, R.; Zák, Z.; Trottier, J.; Janousek, Z.; Viehe, H.G. Tetrahedron Lett. 1993, 34, 8341.
Sample Availability: Available from the author.
© 1997 MDPI. All rights reserved