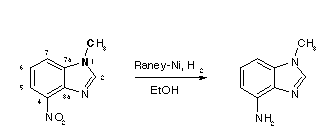
Milata Viktor*, Dusan Ilavsky and Josef Salon
Department of Organic Chemistry, Faculty of
Chemical Technology, Slovak University of Technology,
Radlinskeho 9, SK-812 37 Bratislava, Slovak Republic.
Tel.
00421-7-368 560, 395 410, Fax 00421-7-368 560 ([email protected]
or [email protected])
Received: 7 May 1997 / Published: 26 May 1997
Heterocyclic amines [1] can be prepared from the corresponding nitro-group containing compounds by catalytic or chemical reduction [2].
A solution of 4-nitro-1-methylbenzimidazole (1.77 g, 10 mmol) in ethanol (100 ml) was subjected to hydrogenation with Raney-nickel (prepared from Raney alloy, 2 g ) and hydrogen (120 kPa, 660 ml, 30 mmol). Careful filtration, immediate evaporation on a rotary evaporator and recrystallization (mixture of toluene -heptane) in the presence of charcoal gave 4-amino-1-methylbenzimidazole as orange crystals (1.35 g, 92%).
M.p. 125-127 deg.C.
1H NMR (CDCl3): 7.73 (s, 1H, H-2), 7.11 (t, J=7.7Hz, 1H, H-6), 6.74 (dd, J=7.7 and 0.8 Hz, 1H, H-7), 6.51 (dd, J=7.7 and 0.8 Hz, 1H, H-5), 4.27 (bs, 2H, NH2), 3.77 (s, 3H, NMe).
13C NMR (CDCl3): 141.1 (C-2), 138.8 (C-7a), 135.2 (C-4), 132.5 (C-3a), 124.0 (C-6), 105.8 (C-5), 99.0 (C-7), 31.0 (NMe).
IR (cm-1, KBr): 3424, 3370, 3304, 3186, 3090, 2941, 1638, 1601, 1496, 1343, 1264, 1040, 781, 729.
UV ([lambda]max/log [epsilon], methanol): 222/3.48, 268/2.90, 290/2.78.
Anal. calc. for C8H9N3 (147.18): C 65.13, H 5.98, N 28.72; found: C 65.29, H 6.16, N 28.66.
Acknowledgements: The authors would like to thank Slovak Grant Agency for financial support (No. 95/5195/202) and Dr. Ch. Hammetner (TU Vienna) for the measurement of 2D HETCOR spectra.
References and Notes
1. Mundy, B. P.; Ellerd, M. Name Reactions and Reagents in Organic Synthesis, J. Wiley, New York, 1988.
2. Milata, V. 1-Methyl-X-nitro and aminobenzimidazoles. A review to be submitted.
Sample Availability: Available from the author.©1997 MDPI. All rights reserved