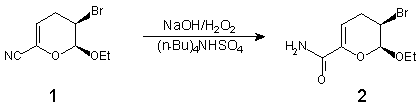
Institute of Organic Chemistry, University of Lausanne, BCH, CH-1015
Lausanne-Dorigny, Switzerland.
Fax: ++ 41 21 692 3955 ([email protected])
Received: 20 June 1997 / Published: 4 July 1997
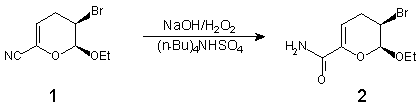
Scheme
The amide 2 [1] was prepared by hydrolysis of the nitrile 1 according to the reported procedure [2].
To a cooled (0deg.C) solution of 1 (232 mg, 1 mmol) in CH2Cl2 (1 ml) was added successively hydrogen peroxide (30%, 0.5 ml), tetra-(n-butyl)ammonium hydrogen sulfate (70 mg) and an aqueous solution of sodium hydroxide (20%, 0.38 ml). The reaction mixture was allowed to warm to room temperature. Methylenechloride (15 ml) was added after 1 h. The organic layer was separated, washed with brine, dried (Na2SO4) and concentrated in vacuo. The residue (0.25 g) was flash chromatographed (AcOEt/Hexane = 3:1) to afford 200 mg (80%) of 2.
Rf (AcOEt): 0.50.
M. p. 141-142 deg.C.
IR (cm-1 , KBr): 3400vs, 3260vs, 1674vs, 1635vs, 1610vs, 1410vs, 975vs, 847s, 810s, 765s.
1H-NMR (CDCl3): 6.33 (br, 1H, NH); 6.26 (br, 1H, NH); 6.06 (dd, J = 5.2, 3.0, 1H, H-5); 5.16 (d, J = 2.1, 1H, H-2), 4.12 (ddd, J = 10.4, 6.3, 2.1, 1H, H-3); 3.87 and 3.71 (2x dq, J = 9.7, 7.1, OCH2Me), 2.77 (ddd, J = 18.2, 10.4, 3.0, 1H, H-4); 2.63 (ddd, J = 18.2, 6.3, 5.2, 1H, H-4); 1.26 (t, J = 7.1, 3H, Me).
13C-NMR (CDCl3): 163.9 (CONH2), 141.5 (C-6), 107.5 (C-5), 97.6 (C-2), 65.2 (OCH2Me), 43.1 (C-3), 27.8 (C-4), 14.8 (OCH2CH3).
CI-MS: 269/267 (M+NH4+, 82/74), 253/251 (M+2, 9/9), 252/250 (M+H+, 87/87), 170 (M-Br, 100), 152/150 (7/7), 125 (31), 124 (41), 99 (9), 97 (21), 85 (11), 77 (31).
Acknowledgment: We thank the Swiss National Foundation for financial support.
References
1. Zhuo, J.-C.; Wyler, H. Helv. Chim. Acta 1995, 78, 151.
2. Cacchi, S.; Misiti, D.; La Torre, F. Synthesis 1980, 243.
Sample Availability: Available from MDPI, 0.1g, MDPI 12539.
© 1997 MDPI. All rights reserved