Scheme
The DASGroup, Inc., 1732 Lyter Drive, 2nd Floor, Johnstown, Pennsylvania 15905-1206, USA, and Department of Chemistry, University of Toledo, Toledo, Ohio 43606-3390, USA. Tel. +1 814 255 7859, Fax +1 814 255 3517, Email [email protected]
Received: 29 September 1997 / Published: 31 October 1997
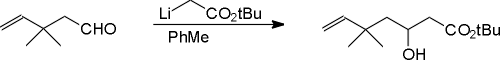
As part of our continuing studies of stereocontrol during the intramolecular Diels-Alder (IMDA) reaction leading to medium rings [1-3], we have targeted poitediol [4] as a structure of interest accessible through this technology. Construction of a suitable IMDA trienone for model studies began with the preparation of the title compound from the known aldehyde [5]. Reformatsky reaction of the aldehyde with methyl bromoacetate in benzene or dimethoxymethane with or without mercuric chloride as a catalyst could not be accomplished. However, alkylation with lithio tert-butylacetate in toluene at 0 �C [6] provided the b-hydroxy ester in 54% unoptimized yield.
To a solution of lithio tert-butyl acetate (55 mg, 0.45 mmol) in dry toluene (1 ml) under Ar at room temperature, the aldehyde (43 mg, 0.38 mmol) in toluene (1 ml) was added. After 30 minutes, the reaction was quenched with sat aq Na2SO4 and concentrated to give, after flash chromatography (4 : 1 pet ether : ether), tert-butyl-3-hydroxy-5,5-dimethyl-6-heptenoate as a colorless oil, 48 mg, in 54 percent yield.
1H NMR (CDCl3): d 5.48 (dd, J = 17.6, 10.7Hz, 1H), 4.95 (dd, J = 10.7, 1.3Hz, 1H), 4.92 (dd, J = 17.6, 1.3Hz, 1H), 4.03 (m, 1H), 2.29 (d, J = 6.3Hz, 2H), 1.6-1.3 (m, 2H), 1.41 (s, 9H), 1.03 (s, 6H).
IR (CH2Cl2): 3560, 2960, 1720, 1375, 1155.
MS (m/e): 213, 172, 154 (100), 128, 102, 94, 84, 70, 50.
HRMS: calc. for C9H16O3 (M - C4H9): 172.1099; found: 172.1099.Sample Availability: No sample available.
© 1997 MDPI. All rights reserved