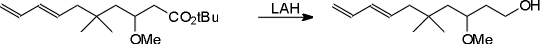
Scheme
The DASGroup, Inc., 1732 Lyter Drive, 2nd Floor, Johnstown, Pennsylvania 15905-1206, USA, and Department of Chemistry, University of Toledo, Toledo, Ohio 43606-3390, USA. Tel. +1 814 255 7859, Fax +1 814 255 3517, Email [email protected]
Received: 29 September 1997 / Published: 31 October 1997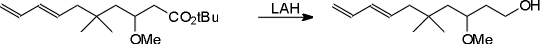
To a slurry of LAH (65 mg, 1.7 mmol) in ether (4 ml) at 0 ÉC under Ar, the diene ester [1] (487 mg, 1.7 mmol) in ether (5 ml) was added dropwise. After addition was complete, the reaction was quenched with sat aq Na2SO4. The solution was filtered and the solids were washed with ether (5 x 10 ml). The combined organic layers were dried over Na2SO4 and concentrated to give, after flash chromatography (1 : 1 pet ether : ether) the title alcohol as a colorless oil, 354 mg, in 97 percent yield.
1H NMR (CDCl3): d: 6.29 (dt, J = 16.9, 10.3Hz, 1H), 6.01 (dd, J = 15.3, 10.7Hz, 1H), 5.71 (dt, J = 15.3, 7.5Hz, 1H), 5.12 (d, J = 16.9Hz, 1H), 4.93 (d, J = 10.3Hz, 1H), 3.81 (m, 1H), 3.69 (m, 1H), 3.48 (m, 1H), 3.30 (s, 3H), 2.37 (bm, 2H), 1.99 (d, J = 7.6Hz, 2H), 1.88 (m, 1H), 1.60 (m, 2H), 1.28 (dd, J - 14.7, 4.0Hz, 1H), 0.90 (s, 3H), 0.88 (s, 3H).
IR (CH2Cl2): 3610, 3490, 2930, 1645, 1600, 1460, 1420, 1385, 1075, 1005, 895.
MS (m/e): 212 (M+, 8), 197, 180, 167, 143, 136, 124, 108, 89 (100).
HRMS: calc. for C13H24O2: 212.1776; found: 212.1774.Sample Availability: No sample available.
© 1997 MDPI. All rights reserved