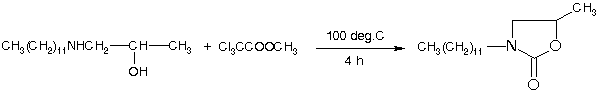
Institute of Chemistry, Slovak Academy of Sciences, Dubravska cesta 9,
SK-84238 Bratislava, Slovak Republic.
Tel. +421-7-378-2540, Fax +421-7-375565 ([email protected])
Received: 28 October 1997 / Published: 18 November 1997
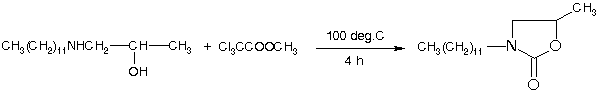
2-Oxazolidinones can be synthesized from appropriate beta-amino alcohols by the action of methyl or ethyl trichloroacetate [1]. Urea may also be used as the carbonyl component as may a variety of other reagents [2].
A mixture of 1-dodecylamino-2-propanol (4.86 g, 20 mmol) and methyl trichloroacetate (4.13 g, 23.3 mmol) was heated at 100 deg.C for 4 h. Ethylene dichloride (30 ml) was added to the resulting red liquid and this was washed with 2N HCl and water. The organic layer was dried and decolourized with charcoal. After evaporation of the solvent, the crude product was recrystallized from hexane to afford the title compound (2.21 g, 41%) as white crystals.
M.p. 40-42 deg.C.
TLC (Hexane/EtOAc 2:1): Rf 0.42.
1H-NMR (CDCl3): 4.62 (m, J=8.4 and 6.9 and 6.3 Hz, 1H, H-5); 3.63 (t, J=8.4 Hz, 1H, H-4); 3.23 (m, 2H, CH2N); 3.10 (dd, J=8.4 and 6.9 Hz, 1H, H-4'); 1.53 (m, 2H, CH2CH2N); 1.42 (d, J=6.3 Hz, 3H, CH3); 1.30 (bs, 4H, 2 x CH2); 1.26 (s, 14H, 7 x CH2); 0.88 (t, J=6.7 Hz, 3H, CH3).
13C-NMR (CDCl3): 158.1 (C=O), 69.8 (C-5), 51.3 (C-4), 44.1 (CH2N), 31.9, 29.6, 29.5, 29.4, 29.3, 27.4, 26.7, and 22.7 (the other CH2 groups), 20.8 (CH3 at C-5), 13.1 (CH3).
Anal. calc. for C16H31NO2 (269.43): C 71.33, H 11.60, N 5.20; found: C 71.15, H 11.69, N 5.28.
Acknowledgments: The authors would like to thank Scientific Grant Agency (VEGA, project No. 2/4144/97) for financial support.
References and Notes
1. Lesher, G. Y.; Surrey, A. R. J. Am. Chem. Soc. 1955, 77,
636.
2. Dyen, M. E.; Swern, D. Chem. Rev. 1967, 67, 197.&
Sample availability: Available from the authors and MDPI, MDPI Reg. No.13742.
© 1997 MDPI. All rights reserved