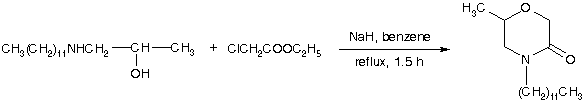
Institute of Chemistry, Slovak Academy of Sciences, Dubravska cesta 9,
SK-84238 Bratislava, Slovak Republic.
Tel. +421-7-378-2540, Fax +421-7-375565 ([email protected])
Received: 28 October 1997 / Published: 18 November 1997
2-Morpholones may be synthesized from the corresponding beta-amino alcohols by heating with an alpha-halo ester [1]. Several alternative methods have also been described [2].
1-Dodecylamino-2-propanol (4.86 g, 20 mmol) was added to a stirred suspension of NaH (0.52 g, 21.5 mmol) in dry benzene (20 ml). The resulting mixture was cooled (ice) and ethyl chloroacetate (2.45 g, 20 mmol) was added during 15 min followed by stirring at room temperature for 1 h and for an additional 1h under reflux. After cooling, ether (50 ml) was added and the mixture was washed with 2 N HCl and water. The organic layer was dried and the solvent evaporated to give a crude product. Recrystallization from hexane afforded the title compound (2.6 g, 46%) as white crystals.
M.p. 37-39 deg.C.
TLC (Hexane/EtOAc 2:1): Rf 0.25.
1H-NMR (CDCl3): 4.18 (ABq, J=16.5 Hz, 2H, H-2); 3.87 (m, J=9.8 and 6.3 and 3.3 Hz, 1H, H-6); 3.40 (m, 1H, CH2N); 3.31 (m, 1H, CH2N); 3.25 (dd, J=12.0 and 9.8 Hz, 1H, H-5); 3.12 (dd, J=12.0 and 3.3 Hz, 1H, H-5'); 1.54 (m, 2H, CH2CH2N); 1.27 (d, J=6.3 Hz, 3H, CH3); 1.26 (s, 18H, 9 x CH2); 0.88 (t, J=6.4 Hz, 3H, CH3).
13C-NMR (CDCl3): 166.5 (C=O), 69.6 (C-6), 67.8 (C-2), 52.4 (C-5), 46.5 (CH2N), 31.9, 29.6, 29.4, 26.9, 22.7 (the other CH2 groups), 18.3 (CH3 at C-6), 14.1 (CH3).
Anal. calc. for C17H33NO2 (283.45): C 72.04, H 11.73, N 4.94; found: C 72.19, H 11.82, N 4.90.
Acknowledgments: The authors would like to thank Scientific Grant Agency (VEGA, project No. 2/4144/97) for financial support.
References and Notes
1. Clarke, F. H. J. Org. Chem. 1962, 27, 3251.
2. Sainsbury, M. in Coffey, S. (Ed.), Rodd's Chemistry of Carbon
Compounds,
Vol. IV, Part H, Elsevier, Amsterdam, 1978, p. 427.
Sample availability: Available from the authors and MDPI, MDPI Reg. No.13732.
© 1997 MDPI. All rights reserved