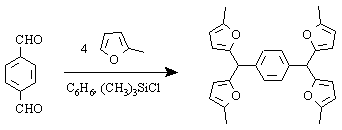
Molecules 1998, 3, M45
a
,a,a',a'- Tetra(5-methylfur-2-yl)-p-xyleneAndrey V. Gutnov
North-Ossetian State University, RUS-362040 Vladikavkaz, Russian Federation.
Fax: +7 867 227 3216 (E-mail: [email protected])
Received: 22 December 1997 / Published: 25 January 1998
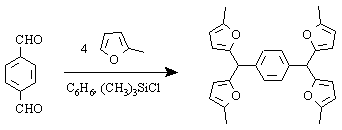
See also [1]. To a solution of terephtalaldehyde (2.7g, 20 mmol) and sylvan (8 ml, 100mmol) in 50 ml of benzene 3 ml of Me3SiCl was added at room temperature. The mixture warmed up to 50-60deg.C within 15 min and a water layer separated. After standing for an additional 15 min, the organic layer was quickly washed with NaHCO3 solution, separated from the water layer and evaporated to dryness. The residue was recrystallized twice from dioxane. Yield 5.14g (60.25%) of white powder.
M.p. 155-156 deg.C.
1
H-NMR (CDCl3): 7.1 (d, 4H, HAr); 5.71 (d, 8H, 3,4-HFur); 3.62 (s, 1H, CH); 2.17 (s, 12H, CH3) ppm.Anal. calc. for C28H26O4 ( 426.51): C 78.85, H 6.14; found: C 78.96, H 6.23.
Acknowledgment: The author would like to thank Dr. Vladimir T. Abaev and Dr. Alexander V. Butin for their support.
References and Notes
1. Gutnov, A. V.; Abaev, V. T.; Butin, A. V.; Zavodnik, V. E.; Kul'nevich, V. G. Khim. Geterotsikl. Soedin. 1996, 12, 162-167.
Sample availability: 550 mg from the authors.
©1998 MDPI. All rights reserved