G. Boyer* and J.P. Galy
ESA 6009, Faculté des Sciences
et Techniques, case 552, Avenue Escadrille Normandie-Niemen, 13397 Marseille
Cedex 20, France
Fax & Tel.: 04 91 28 83 23,
E-mail : [email protected]
Received: 14 July 1998 / Published: 29 July 1998
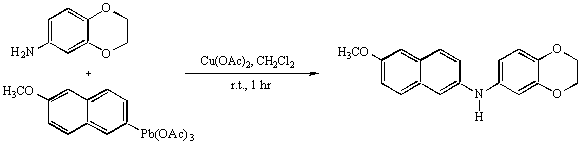
The general part of the experimental section [1] on organometallic substitution on arylamines has been described elsewhere. The N-aryl amine is a precursor of the phenothiazine drugs.
3,4-Ethylenedioxyaniline (0.5g, 3.3 mmol, 1 equ.), 6-methoxy-2-naphthyl lead triacetate [2], (1.95g, 3.6 mmol, 1.1 equ.), copper diacetate (54 mg, 0.3 mmol, 0.1 equ.), were stirred in anhydrous methylene chloride (30ml) under nitrogen during 1 hour at room temperature. The red solution was evaporated and the residue was chromatographed on silicagel with CH2Cl2 as the eluant. The titled N-arylamine was recovered as a white powder, 0.84g (83% yield).
M.p. 102 deg.C.
1H-NMR (DMSO-d6): 7.95 (s, NH), 7.65 (d, J = 8.8, 1H), 7.57 (d, J = 8.9, 1H), 7.28 (d, J = 2.1, 1H), 7.17 (dd, J = 8.7, 2.3, 1H), 7.03 (dd, J = 8.9, 2.6, 1H), 6.78 (d, J = 8.2, 1H), 6.65 (d, J = 2.5, 1H), 6.64 (dd, J = 8.2, 2.5, 1H), 4.22 (m, 4H), 3.82 (s, 3H).
13C-NMR (DMSO-d6): 155.14, 143.66, 140.53, 137.52, 137.52, 137.39, 129.75, 128.76, 127.70, 127.62, 120.11, 118.58, 117.58, 117.43, 111.57, 108.75, 106.68, 106.20, 64.35, 63.92, 55.10.
Anal calc. for C19H17NO3 (307.30): C 74.25, H 5.57, N 4.56; found: C, 74.10, H 5.79, N 4.81.
References and Notes:
1. Boyer, G.; Galy, J. P.; Barbe J. Heterocycles 1995, 41, 487.
2. Kozyrod, R. P.; Morgan, J.; Pinhey, J. T. Aust. J. Chem. 1985, 38, 1147.
Sample Availability: Available from MDPI.
©1998 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/