Wei-Min Chen1 and Long-Mei Zeng2
1 Institute of Pharmaceutical Sciences, The First Military
Medical University, Guangzhou 510515, P. R. China. Tel.& Fax +86-20-87705671([email protected]).
2Department of Chemistry, Zhongshan University, Guangzhou
510275, P. R. China. Tel. +86-20-84185447 ([email protected])
Received: 14 July 1998 / Published: 29 July 1998

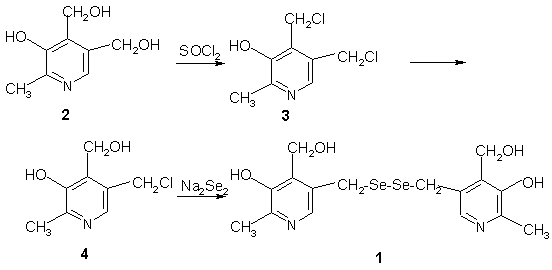
The intermediate 3 was synthesized utilizing the methodology
of Deng [1]. A mixture of 3 (3.4g, 14mmol) in 60ml of H2O
was stirred at 60-65deg.C for 1.4h and cooled to room temperature. Then
25ml of Na2Se2 solution (19mmol) was added dropwise.
After adjustment to pH 8, the mixture was stirred at 60deg.C for 2h and
cooled to room temperature. The precipitate was filtered off and washed
with water. The solid was solved in 20ml of 10% HCl, and stirred for 0.5h,
filtered and washed with 10% HCl. The mother liquor was adjusted to pH
8 with 40% NaOH to give a precipitate which was filtered off and washed
with water. Yellow solid 3,3'-[diselenobis(methylene)]bis[5-hydroxy-6-methyl-4-pyridine-methanol
(1) 2.5g (77.3%).
Mp:192-3deg.C.
IR(KBr) n (cm-1): 3100 (-OH), 2706, 2628, 1560, 1405, 1236, 1039, 772, 716.
1H-NMR (DMSO-d6) dH (ppm): 2.32 (s, 6H, -CH3), 3.98 (s, 4H, -CH2Se-), 4.75 (s, 4H, -CH2O), 7.70 (s, 2H, H-2).
MS (m/z,%): 462 (M+, 2%) 231, 213, 152, 136, 122, 106, 94, 77, 66, 53, 39 (100%).
Anal.(C16H20N2Se2O4) C, H, N.
Acknowledgment: This research was supported by the Natural Sciences Foundation of Guangdong Province.
Reference
1. Deng L. Chinese Journal of Pharmaceuticals 1996, 27(8), 342.
Sample Availability: Available from MDPI.
©1998 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/