Pontificia Universidad Javeriana, Grupo de Investigación Fitoquímica
GIFUJ, Santafé de Bogotá, Colombia.
Tel 2887740, fax 2850503, E-mail [email protected]
Received: 26 October 1998 / Published: 16 April 1999

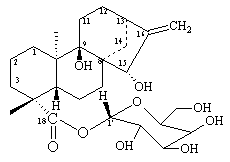
From leaves and flowers of Ageratina vacciniaefolia we have isolated several compounds: a flavonoid, a diterpene and a new compound identified as (-)-b-D-18–glucopiranosyl-9,15-dihydroxy kaurenoate (see the formula). The structure was established by using 1HNMR, 13CNMR, COSY, NOESY and HMBC spectroscopic techniques (See Table 1) [1,7].
The ethanolic extract of leaves and flowers of A. vacciniaefolia yielded white crystals after column chromatography eluted with CH2Cl2, EtOAc and mixtures of these solvents.
M.p. 218-220°C.
[a]20D = -100 (MeOH).
DCI-MS [isobutane] m/z: (M*+H)+ 497; 479 (M-18); 377; 337; 335; 317; 299; 271; 230; 187; 163; 145 (100%); 127.
1HNMR (400 MHz, C5D5N): d 1.15 (dt, 1H, CH2, J=4.3; 12.3); 1.33 (s, 3H, CH3); 1.53 (s, 3H, CH3); 1.55 -1.70 (m, 2H, CH2); 1.58 - 1.60 (m, 1H, CH2); 1.66 (m, 1H, CH2); 1.87 (ddd, 1H, CH2; J=12.0; 3.8); 2.17 - 2.18 (m, 2H, CH2); 2.25 (m, 1H, CH); 2.28 (m, 1H, CH2); 2.30 (dq, 1H, CH2; J=3.2; 12.4); 2.33 (m, 1H, CH2); 2.44 (m, 1H, CH2); 2.50 (dd, 1H, CH2; J=12.0; 13.0); 2.75 (bs, 1H, CH); 4.06 (ddd, 1H, CH; J=2.4; 4.3; 9.5); 4.24 (bt, 1H, CH; J=7.8); 4.28 (t, 1H, CH; J=8.0); 4.35 (t, 1H, CH; J=8.9); 4.40 (dd, 1H, CH2; J=4.3; 11.9); 4.46 (dd, 1H, CH2; J=2.4; 11.9); 5.31 (bs, 1H, CH); 5.20/5.46 (bs, 2H, CH2); 6.33 (d, 1H, CH; J=7.8, anomeric proton).
13C NMR (100 MHz; C5D5N): d 18.6 (-CH3, C20); 20.3 (-CH2, C1); 22.5 (-CH2, C6); 29.4 (-CH3, C18); 30.0 (-CH2, C11); 31.7 (-CH2, C7); 33.3 (CH2, C1); 34.9 (-CH2, C12); 37.7 (-CH2, C14); 39.0 (-CH2, C3); 42.4 (-CH, C13); 45.0 (-C-, C4); 45.7 (-C-, C10); 51.4 (-CH, C5); 54.3 (-C-, C8); 62.7 (-CH2-O-, C6'); 71.6 (-CH, C4'); 74.6 (-CH, C2'); 77.4 (-C-OH, C9); 78.0 (-CH-OH, C15); 79.7 (-CH, C3'); 79.9 (-CH, C5'); 96.3 (-CH, C1'); 107.9 (=CH2, C17); 162.3 (=C, C16); 178.0 (-COO, C19). For details, see table 1.
Table 1. Direct and long range NMR correlations and their assignments
[2-6].
|
|
|
|
|
|
| 19 | COO- | 178.0 | 6.33 2.25 1.33 | |
| 16 | C = | 162.3 | 5.46 5.20 2.50 1.87 | |
| 17 | CH2= | 107.9 | 5.46, 5.20 s,b | |
| CH - 1’ | 96.3 | 6.33 d (7.8) | 4.24 | |
| CH - 5’ | 79.9 | 4.06 ddd (2.4, 4.3, 9.5) | 4.35 | |
| CH - 3’ | 79.7 | 4.28 t (8.0) | 4.35 | |
| 15 | CH | 78.0 | 5.31 s,b | 5.46 5.20 2.5 |
| 9 | C - O | 77.4 | 5.31 5.22 2.17 1.87, 1.6 1.53 | |
| CH - 2’ | 74.6 | 4.24 bt (7.8) | 4.28 | |
| CH - 4’ | 71.6 | 4.35 t (8.9) | 4.18 4.28 | |
| CH2-O-6’ | 62.7 | 4.46 dd (2.4 ;11.9) | 4.35 | |
| 4.40 dd (4.3 ;11.9) | ||||
| 8 | C | 54.3 | 5.22(OH),
2.75 2.5
2.33 2.17 1.87 |
|
| 5 | CH | 51.4 | 2.25 m. | 2.66, 2.44, 2.17 1.66, 153, 1.33 |
| 10 | C | 45.7 | 2.25 4.66 1.53 | |
| 4 | C | 45.0 | 2.44 2.25 1.33 1.15 | |
| 13 | CH | 42.4 | 2.75 s.b | 5.46 5.20 2.17 1.87 |
| 3 | CH2 | 39.0 | 2.44 m |
|
| 1.15 dt (4.3 ; 12.3) | ||||
| 14 | CH2 | 37.7 | 2.50 dd (12, 13) | 531 2.17 |
| 1.87 ddd (12, 3.8) | ||||
| 12 | CH2 | 34.9 | 1.55 - 1.7 m (2H) | 2.5 2.17 |
| 1 | CH2 | 33.3 | 2.33 m | 2.44 1.53 |
| 1.66 m | ||||
| 7 | CH2 | 31.7 | 2.18 d | 2.66 2.25 1.87 |
| 11 | CH2 | 30.0 | 2.17 m | 5.22 (OH) 1.78 |
| 18 | CH3 | 29.4 | 1.33 s | 2.25 1.15 |
| 6 | CH2 | 22.5 | 2.30 dq ( 3.2 ; 12.4) | 2.25 2.17 |
| 2.28 m | ||||
| 1 | CH2 | 20.3 | 2.33 m 1.60 m | 1.15 2.3 |
| 1.58 m | ||||
| 20 | CH3 | 18.6 | 1.53 s | 2.17 |
References
1. Hasan, C. M., Healey, T. M. and Waterman P. T. Phytochemistry1982,
21,
1365.
2. Demetzos, C.; Harvala, C.; Phillianos, S.; Manol Skaltsounis, A.
J.
Nat. Prod. 1991, 53, 1365.
3. Torrenegra, R.; Pedrozo, J.; Robles, J.; Waibel, R.; Achenbach,
H. Phytochemistry 1992, 31, 2415.
4. Konzi, S. A.; McChesney, J. D. J. Nat. Prod. 1991,
54,
483.
5. Aranda, G.; El Kortbi, M.S.; Lallemand, J. Y. Tetrahedron 1991,
47,
8339.
6. Konig, W.A.; Lutz, S.; Wenz, G. Angew. Chem. 1988,
100
, 989.
7. Breitmaier Eberhard, Structure Elucidation by NMR in Organic
Chemistry, John Wiley & Sons, New York (1993).
Sample Availability: Available from the authors and from MDPI.
MDPI
16328
©1999 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/