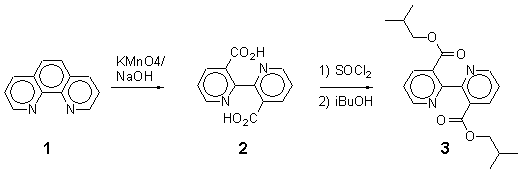
Diisobutyl 2,2'-Bipyridine-3,3'-dicarboxylate
T. Ben Hadda, N. Sam* and A. Zahidi
Laboratoire de Chimie d'Activation Molulaire, Faculté des Sciences d'Oujda, Université Mohamed Ier, Route Sidi Maafa, 60000 Oujda, Maroc. E-mail: [email protected]
Received: 28 June 1999 / Accepted: 2 July 1999 / Published: 20 August 1999

M.p. 88-89 °C.
IR (KBr, cm-1): 1710, 1560, 1440, 1265.
1H-NMR (250 MHz, CDCl3): 8.74 (dd; 2H, H6/6', J = 4.8 and 1.6 Hz); 8.37 (dd, 2H, H4/4', J = 7.9 and 1.6 Hz); 7.44 (dd, 2H, H5/5', J = 7.9 and 4.8 Hz), 3.86 (d, 4H, 2CH2, J = 6.6 Hz), 1.68 (m, 2H, 2CH); 0.76 (d, 12H, 4CH3, J = 6.6 Hz).
Anal. Cal. for C20H24N2O4 (356.42): C 67.42, H 6.74, N 7.86; found : C 67.55, H 6.82, N 7.87.
Acknowledgement: We gratefully acknowledge the assistance from Laboratory of Organometalic and Catalyst, UMR 6509 CNRS, Campus Beaulieu, University of Rennes I.
References
1. Forostyan, Y. N.; Efimova, E. I.; Varvanskayae, M. N.; Tetreshchenkoe, L. G. Chem. Heterocycl. Compd. (Engl.Transl.) 1972, 8, 1492.
2. Mukkala, V. M.; Kibaut, J. J. Helv. Chem. Acta. 1992, 75, 1578-1592.
Sample Availability: Available from the authors and from MDPI. MDPI 17154.
©1999 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/