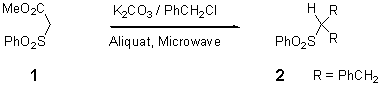
1,3-Diphenyl-2-phenylsulfonylpropane
Mohammed Ramdania*, André Loupyb and Alain Petitb
aLaboratoire de chimie organique
et physique, Université Mohamed 1er, Faculté des
sciences, Oujda, Maroc. Fax (212)6 74 47 49 E-mail: [email protected]
bLaboratoire des réactions
sélectives sur supports, CNRS, UA 478, ICMO, Université Paris
XI, bâtiment 410, 91405 Orsay cédex, France. Fax (33) 1 69
15 46 79 E-mail: [email protected]
Received: 28 June 1999 / Accepted: 2
July 1999 / Published: 20 August 1999
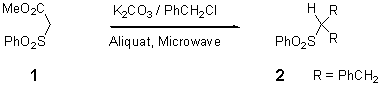
The product 2 was prepared from methyl phenylsulfonyl acetate in situ by the solid-liquid PTC conditions without solvent [1-5]. A mixture of the ester 1 (0.535 g, 2.5 mmol), benzyl chloride (0.791 g, 6.25 mmol), K2CO3 (0.862 g, 6.25 mmol) and 10 % of phase transfer catalyst Aliquat (0.1 g, 0.25 mmol) was placed in a pyrex tube which was then introduced into a Maxidigest MX 350 Prolabo microwave monomode reactor fitted with a rotational system. An approximate temperature of 130°C was measured at the end of the irradiation time (6 min with 140 w as irradiation power).The mixture was allowed to cool to ambient temperature. After dilution with ethyl acetate (30 ml) and subsequent filtration through FlorisilTM, the organic product was analysed by GC (using an internal standard) using a CPSIl, SCB - 25 m capillary column on a Carlo Erba CG 6000 (flame ionisation; gas carrier: He (70 Kpa); Temperatures (injector and detector): 280°C. Programmation in temperature: 200°C (1h) and 200-280°C (10°C / min)) and purified by recrystallization in diethyl ether (yield: 64 % of 2).
1H NMR (CDCl3): 2.8-2.9 (dd, 2H); 3.22-3.36 (dd, 2H); 3.58-3.68 (m, 1H); 6.8-7.2 (m, 10H); 7.45-7.9 (m, 5H).
IR (Nujol): 1310 and 1140 cm-1 (SO2).
MS (IC-NH3, m / z): 354 (M+ + 18) / 100 %.
References
1. Loupy, A.; Pigeon, P.; Ramdani, M.;
Jacquault, P. J. Chem. Research(S) 1993, 36-37.
2. Barnier, J. P.; Loupy, A.; Pigeon,
P.; Ramdani, M.; Jacquault, P. J. Chem. Soc. Perkin Trans. 1 1993,
397-398.
3. (a) Krapcho, A. P. Synthesis1982,
805. (b) Krapcho, A. P. Synthesis 1982, 893.
4. Fujii, M.; Nakamura, K.; Mekata, H.;
Oka, S.; Ohno, A. Bull. Chem. Soc. Jpn. 1988, 61,
495-500.
5. Yuliang, W.; Yaozhong, J. Synth.
Commun. 1992, 22, 2287-2291.
Sample Availability: Available from the authors.
©1999 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/