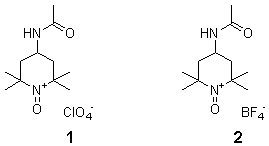
Explosion of 4-Acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium Perchlorate and Replacement by Tetrafluoroborate
James M. Bobbitt
Department of Chemistry, University of Connecticut, 215 Glenbrook Road, Room 151, Storrs, CT 06269-4060, USA, voice phone: 860-486-6601, fax: 860-486-2981, E-mail: [email protected]
Received: 17 June 1999 / Published: 5 September 1999

In December, 1998, I published a paper (J. Org. Chem. 1998 63 , 9367) describing the synthesis and reactions of two oxoammonium salts, 4-acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium perchlorate (1) and tetrafluoroborate (2). These salts are non-heavy metal, colorimetric oxidizing agents for the conversion of alcohols to aldehydes or ketones in essentially quantitative yields with minimal work-up procedures [1,2].
Almost all of the experimental procedures described were related to the perchlorate 1, although the oxidants 1 and 2 seemed to have nearly identical properties. The air-dried perchlorate has been used for about six years and was thought to be quite stable. However, when an 8 g. sample was dried over P2O5 under high vacuum at 55°C., it detonated. Damage was minimal, but the explosion was quite violent.
Those who might have prepared a supply of the perchlorate are urged to destroy it by dissolving it in water and reducing it with ethanol and NaHCO3 as described in the paper.
The tetrafluoroborate 2 is quite stable up to at least 95°C. under the conditions described above and has the same outstanding oxidizing properties as the perchlorate.
Acknowledgment: Partial financial support was provided by the National Science Foundation (Grant CTS-9632391).
References
1. Bobbitt, J. M. Oxoammonium Salts. 6. 4-Acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium Perchlorate: A Stable and Convenient Reagent for the Oxidation of Alcohols. Silica Gel Catalysis. J. Org. Chem. 1998, 63 , 9367-9374.
2. See ref. 1 and relevant papers cited therein.
Sample Availability: 4-Acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate (2) is available from MDPI, MDPI 17072.
©1999 by the authors. Reproduction of this article, by any means, is permitted for noncommercial purposes. Molecules is published online (www.mdpi.org/molecules/) by MDPI in Switzerland.