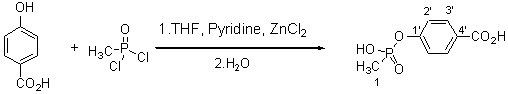
4-Carboxylphenyl Methylphosphonate
Jianhua Yu, Dejun Ma, Qibin Huang, Yufeng Jia and Yundu Deng
Beijing Institute of Pharmaceutical chemistry, Box 1044-301, Beijing, 102205, P.R.China. Tel: 86-010-69760429-92370, fax: 86-010-69760527, E-mail: [email protected]
Received: 18 August 1999 / Accepted: 28 August 1999 / Published: 8 October 1999
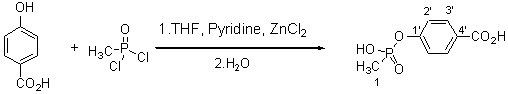
Methylphosphonic acid monoesters have been explored for many different purposes [1, 2]. We have synthesized a novel ester, 4-carboxylphenyl methylphosphonate, in order to construct an artificial recognition system. The compound, as a transition state analogue of the alkaline ester hydrolysis, is able to form multiple hydrogen bonds with the functional monomer and can be used as hapten to generate antibodies that catalytically accelerate the hydrolysis of carboxylic esters.
A solution of p-hydroxy benzoic acid (100g, 0.76mol) in 80ml of THF was slowly added to a mixture of methylphosphonic dichloride [3] (100g, 0.76mol), THF (30ml), pyridine (71g) and ZnCl2 (2.0g, 0.015mol) with stirring. The reaction mixture was heated slowly to boiling and refluxed for 4hr. When cool, it was adjusted to pH 13-14 by the addition of 20% NaOH solution and refluxed for 5 min. The solution was cooled again, acidified to pH 3 and extracted with ethyl ether (3 x 80ml) to remove unreacted p-hydroxy benzoic acid. The aqueous layer was then strongly acidified with concentrated HCl to pH 1. The aqueous phase was extracted with ethyl acetate, separated, dried (MgSO4), concentrated and the product was allowed to crystallize in the refrigerator. If no product appeared in 6-8h, n-hexane was added to the cloud point and the product then crystallized overnight. The solution gave 127.2g product as a white powder, this represents a yield of 78.3% of 4-carboxylphenyl methylphosphonate based on p-hydroxy benzoic acid.
M.p. 202-203°C.
MS (m/z, %): 216 (43, M+), 139 (7, M-77), 138 (78, M-78), 121 (100), 93 (18), 79 (9), 65 (20), 64 (5).
IR (KBr, cm-1): 3300-2550 (-OH), 1687 (C=O), 1604, 1509, 1431, 1169, 1318, 1295 (P-CH3), 1221 (P=O), 947 (P-O-C(aryl)), 863.
1H NMR (500MHz, DMSO-d6, Me4Si): 1.54 (d, 3H, 2JPH=17.5Hz, -CH3), 3.45 (s, 2H, -OH), 7.28 (dd, 2H, 3JHH=8.5Hz, 4JP H=1Hz, 2-H, 6-H), 7.94 (d, 2H, 3JHH=8.5Hz, 3-H, 5-H).
13C NMR (125MHz, DMSO-d6): 12.4 (-CH3, 1JPC =139), 120.5 (C2, C6), 126.6 (C4), 131.3 (C3, C5), 154.5 (C1, 2JPC=7.25), 166.6 (-COOH).
References and Notes
| 1. | Jubrail, R.; Pratt, R. F. Biochemistry1994, 33, 116-125. |
| 2. | Wulff, G.; Schonfeld, R. Adv. Mater. 1998, 10, 957-959. |
| 3. | The compound was synthesized according to the method described in Corbridge, D. E. C. Phosphorus; 3rd. Ed., Elsevier, Amsterdam-Oxford-New York-Tokyo, 1985, 231. |
Sample Availability: Available from the authors in a large quantity and from MDPI. MDPI 17905.
© 1999 MDPI. All rights reserved.
Molecules
website www.mdpi.org/molecules/