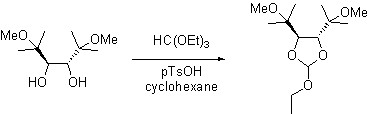
University of Lausanne, ICO-BCH, 1015 Lausanne-Dorigny, Switzerland. Phone : +41 21 692 40 69 E-mail: [email protected]
Received: 28 August 1999 /Accepted:7 September 1999 /Published: 8 October 1999
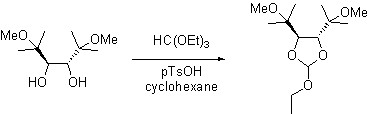
The title compound was prepared from 5 g (89 mmol, 1 eq) of (3R,4R)-2,5-dimethoxy-2,5-dimethyl-3,4-hexanediol [1], 4.4 ml (3.95 g, 27 mmol, 1.1 eq) of triethyl orthoformate, 0.05 g (0.25 mmol, 0.01 eq) of p-toluenesulfonic acid monohydrate in 35 ml of cyclohexane under inert atmosphere. The reaction mixture was stirred for 90 minutes at room temperature followed by distillation for 24 hours at 100°C to remove the formed ethanol. The mixture was allowed to cool to ambient temperature. The crude product was purified by Kugelrohr distillation over 0.5 g of K2CO3 at 170°C under reduced pressure (10 Torr) to give 5.64 g (89%) of the product as a colorless liquid.
1H NMR (CDCl3, 200 MHz): 5.89 (s, 1 H, CH); 4.07 (AB, J = 3.2, 1 H, CHCH); 4.01 (AB, J = 3.2, 1 H, CHCH); 3.66 (q, J = 7.0, 2 H, CH2); 3.23 (s, 3 H, OCH3); 3.22 (s, 3 H, OCH3); 1.13-1.24 (m, 15 H, 5xCH3).
13C NMR (CDCl3, 50 MHz): 117.6 (D, J = 99, CH); 83.4 (D, J = 147, CHCH); 81.7 (D, J = 150, CHCH); 76.3 (S, C); 75.7 (S, C); 61.1 (T, J = 142, CH2); 49.5 (Q, J = 141, OCH3); 49.4 (Q, J = 141, OCH3); 22.0 (Q, CCH3); 21.0 (Q, J = 126, CCH3); 20.3 (Q, CCH3); 19.3 (Q, CCH3); 15.2 (Q, J = 126, CH2CH3)
EI-MS: 261 (15, [M-H]+); 247 (5, [M-Me]+); 217 (20, [M-OEt]+); 189 (70, [M-MeOCMe2]+); 73 (100, [CMe2OMe]+)
References and Notes
1. The diol was prepared using described procedure : Mash, E. A.; Hemperly, S. B.; Nelson, K. A.; Heidt, P. C.; Vandeusen, S. J. Org. Chem. 1990, 55, 2045.
Sample Availability: Samples are not available.
©1999 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/