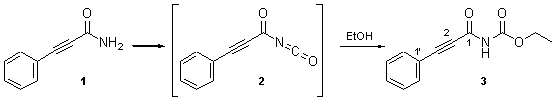
Ethyl N-(3-Phenyl-2-propynoyl)carbamate
Dieter Hubmann and Urs Séquin*
Institut für Organische Chemie der Universität Basel, St. Johanns-Ring 19. CH-4056 Basel, Switzerland. Tel. +41 61 2671110, Fax +41 61 2671103, E-mail: [email protected]
Received: 28 August 1999 / Accepted: 2 September 1999 / Published: 8 October 1999
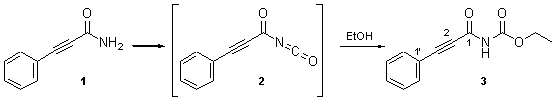
During the synthesis of a series of carboximides using acyl isocyanates [1], the title compound 3 was synthesised in a model reaction from 3-phenyl-2-propynoyl isocyanate (2) and ethanol. Isocyanate 2 was generated in situ from the corresponding carboxamide (1) with oxalyl chloride [2]. Compound 3 had been reported previously [3], but we give here a more detailed characterization.
To 3-phenyl-2-propynamide (1 [3], 492 mg, 3.39 mmol) in dichloromethane (8 ml), oxalyl chloride (407 ml, 4.75 mmol) was added under argon at room temperature. The mixture was heated to reflux for 5 h and then allowed to cool to room temperature. Ethanol (1 ml, 177 mmol) was added and the mixture stirred for 10 min and the solvent removed in vacuo. The residue was chromatographed on SiO2 (40 g, dichloromethane) to give 645 mg (89%) of 3 as a colorless solid.
Colorless prisms (ethanol), m.p. 98.5-100°C ([3]: 100°C).
IR (KBr): 3211; 3100; 2987; 2204; 1768; 1648; 1479; 1358; 1206; 1192; 1066; 769; 696.
1H-NMR (300 MHz, CDCl3): 7.96 (s br, 1H, NH); 7.63 (d, J = 6.8, 2H, H-C(2'), H-C(6')); 7.47 (t,J = 7.4, 1H, H-C(4')); 7.39 (t, J = 7.3, 2H, H-C(3'), H-C(5')); 4.29 (q, J = 7.1, 2H, CH2); 1.34 (t, J = 7.1, 3H, CH3).
13C-NMR (75 MHz, CDCl3): 151.9, 150.5 (2 C=O); 133.1 (C(2'), C(6')); 130.9 (C(4')); 128.6 (C(3'), C(5')); 119.6 (C(1')); 92.3 (C(3)); 81.8 (C(2)); 62.6 (CH2); 14.2 (CH3).
EI-MS (70 eV): 217 (6, M+); 189 (8); 171 (6); 145 (18); 129 (100); 118 (30); 101 (7); 89 (11); 75 (20); 51 (11).
CI-MS (NH3): 235 (10, [M+NH4]+); 218 (100, [M+H]+); 146 (8); 129 (16).
Anal. Calcd for C12H11NO3 (217.22): C 66.35, H 5.10, N 6.45; Found: C 66.19, H 5.03, N 6.36.
Acknowledgement: Financial support by the Schweizerischer Nationalfonds zur Förderung der wissenschaftlichen Forschung (project no. 20-41857.94) is gratefully acknowledged.
References
| 1. | Hubmann, D.; Liechti, C.; Trinks, U.; Traxler, P.; Séquin, U. Synthesis of N-Phenylpyrrole Carboximides. Molecules1999, 4, 151-158. |
| 2. | Speziale, A. J.; Smith, L. R.; Fedder, J. E. The Reaction of Oxalyl Chloride with Amides, IV. Synthesis of Acyl Isocyanates. J. Org. Chem. 1965, 30, 4306-4307. |
| 3. | Ruhemann, S.; Priestley, J. G. The Action of Ethyl Carbamate on Esters of Organic Acids and Mustard Oils. J. Chem. Soc. 1909, 95, 449-456. |
Sample availability: available from the authors and from MDPI. MDPI ID 17924.
©1999 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/