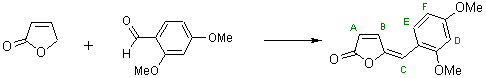
4-(2,4-Dimethoxybenzyliden)-2-butenolide
Lyudmila N. Sorotskayaa, Tatyana Ya. Kaklyuginab and Larisa A. Badovskayac
a Laboratory of Furan Chemistry, bDepartment of Organic Chemistry, c Department of General Chemistry, Kuban State Technological University, Moskovskaya st. 2, Krasnodar 350072, Russia. a E-mail: [email protected], bE-mail:[email protected]
Received: 25 August 1999 / Accepted: 8 September 1999 / Published: 8 October 1999
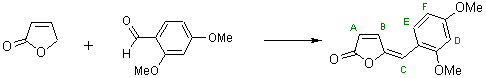
The general part of the experimental section [1] has been presented elsewhere. To a solution of 8.8 g (0.05 mol) of 2,4-dimethoxybenzaldehyde in 100 ml of hot ethanol 2-butenolide (7.6 ml, 0.1 mol) was added. Piperidine (2.5 ml, 0.025 mol) was then added dropwise to the stirred mixture. The reaction mixture was stirred for 3 hours and then left to stand at room temperature for 24 hours. The crystals obtained were filtered off. Cooling of the filtrate in the refrigerator enabled isolation of an additional quantity of the reaction product. Yield of 4-(2,4-dimethoxybenzyliden)-2-butenolide is 9.86 g (85 %).
M.p. 185°C (ethanol).
IR (cm-1): 1650 (C=C); 1745 (C=O), 1790 (C=O).
1H NMR (CDCl3, 250 MHz) : 8.20 (d, 1H, HE, JEF = 8.8 Hz); 7.50 (d, 1H, HB, JBA = 5.2 Hz); 6.58 (dd, 1H, HF, JFE = 8.8 Hz, JFD = 2.6 Hz); 6.50 (br s, 1H, HC, JCA = 0.8 Hz); 6.45 (d, 1H, HD, JDF = 2.6 Hz); 6.10 (dd, 1H, HA, JAB = 5.2 Hz, JAC = 0.8 Hz); 3.86 (d, 3H, OCH3); 3.84 (s, 3H, OCH3).
EI-MS: 232 (M+ 100 %); 217 (21.7); 204 (66.7); 201 (30); 190 (38.3); 189 (83); 175 (20); 158 (83).
Anal. calc. for C13H12O4 (232.23): C 67.23, H 5.21; found C 67.21, H 5.26.
Reference
1. Sorotskaya, L.N.; Badovskaya, L.A.; Kaklyugina, T.Y.; Belen'kij, L.I.; Ignatenko, A.V.; Krutoshikova, A.; Panieva, L.A. Zhurnal Organich. Khimii (Journal of Organic Chemistry - Russian Edition) 1989, 25, 175.
Sample availability: available from authors (Dr. Lyudmila N. Sorotskaya) and MDPI.
©1999 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/