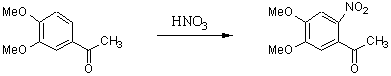
4,5-Dimethoxy-2-nitroacetophenone
Dmitrij S. Zavgorodniy, Alexander V. Butin and Tat'yana A. Stroganova
Research Laboratory of Furan Chemistry, Kuban State Technological University, Moskovskaya 2, Krasnodar, Russian Federation. Phone: +7 8612 55 95 56; E-mail: [email protected], E-mail: [email protected]
Received: 13 September 1999 / Accepted: 20 September 1999 / Published: 8 October 1999
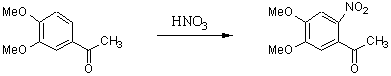
The general part of the experimental section [1] has been presented elsewhere. To solution of 4,5-dimethoxyacetophenone (20.0 g, 110 mmol) in 45 ml of glacial acetic acid, red fuming nitric acid (22.0 ml) was added dropwise with cooling in an ice-water bath. After 20 minutes the reaction mixture was poured into water. Increasing the reaction time caused by-product formation. The precipitate was filtered off, washed with water and recrystallized from ethanol to yield 14.3 g (58 %) 4,5-dimethoxy-2-nitroacetophenone.
M.p. 134°C (ethanol).
1H NMR (CDCl3, 80 MHz): 7.60 (s, 1H, 3-HAr); 6.75 (s, 1H, 6-HAr); 3.93 (s, 6H, OCH3); 2.42 (s, 3H, CH3).
IR (cm-1): 1680 (C=O).
Anal. calc. for C10H11NO5 (225.21): C 53.33, H 4.92; Found: C 53.17, H 5.09.
Reference
1. Gutnov, A.V.; Butin, A.V.; Abaev, V.T.; Krapivin, G.D.; Zavodnik, V.E. Furyl(aryl)alkanes and Their Derivatives.19. Synthesis of Benzofuran Derivatives via 2-Hydroxyaryl-R-(5-methylfur-2-yl)methanes. Reaction of Furan Ring Opening - Benzofuran Ring. Molecules 1999, 4, 204-218.
Sample availability: available from the authors and from MDPI.
©1999 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/