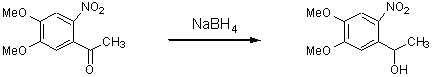
1-(4,5-Dimethoxy-2-nitrophenyl)ethanol
Dmitrij S. Zavgorodniy, Tat'yana A. Stroganova and Alexander V. Butin
Research Laboratory of Furan Chemistry, Kuban State Technological University, Moskovskaya 2, Krasnodar, Russian Federation. Phone: +7 8612 55 95 56; E-mail: [email protected], E-mail: [email protected]
Received: 13 September 1999 / Accepted: 20 September 1999 / Published: 8 October 1999
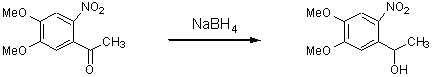
The general part of the experimental section [1] has been presented elsewhere. To a solution of 4,5-dimethoxy-4-nitrobenzophenone (8.0 g, 36 mmol) in 80 ml of ethanol, NaBH4 (0.67 g) was added. After 15 minutes the reaction mixture was acidified with hydrochloric acid (20 %) to pH 6. The precipitate obtained was filtered off and purified by crystallisation from ethanol to yield 5.73 g (70 %) of 1-(4,5-dimethoxy-2-nitrophenyl)ethanol as yellow needles. After dilution of the acidified filtrate with water (100 ml) an additional quantity (1.15 g) of the titled compound was isolated.
M.p. 126°C (ethanol).
IR (cm-1): 3260 (OH).
1H NMR (CDCl3, 80 MHz): 7.53 (s, 1H, 3-HAr); 7.28 (s, 1H, 6-HAr); 5.52 (k, 1H, J=6.3, CH); 3.93 (s, 3H, OCH3); 3.88 (s, 3H, OCH3); 2.56 (broad s., 1H, OH); 1.48 (d, 3H, J=6.3, CH3).
Anal. calc. for C10H13NO5 (227.22): C 52.86, H 5.76; Found: C 52.72, H 5.89.
Reference
1. Gutnov, A.V.; Butin, A.V.; Abaev, V.T.; Krapivin, G.D.; Zavodnik, V.E. Furyl(aryl)alkanes and Their Derivatives.19. Synthesis of Benzofuran Derivatives via 2-Hydroxyaryl-R-(5-methylfur-2-yl)methanes. Reaction of Furan Ring Opening - Benzofuran Ring. Molecules 1999, 4, 204-218.
Sample availability: available from the authors and from MDPI.
©1999 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/