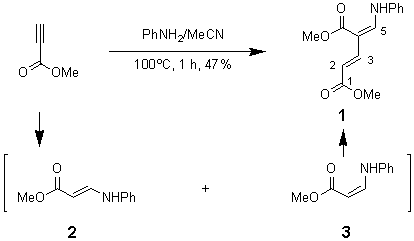
Methyl (2E,4E)-5-Anilino-4-(methoxycarbonyl)penta-2,4-dienoate
Jin-Cong Zhuo
College of Pharmacy, The Ohio State University, 500 West 12th Avenue, Columbus, OH 43210, USA. Fax: (614) 292 2435; E-mail: [email protected]
Received: 17 September 1999 / Accepted: 4 October1999 / Published: 23 November 1999

Method A: A mixture of methyl propiolate (0.42 g, 5 mmol) and aniline (0.47 g, 5 mmol) in MeCN (3 ml) was heated at 100ºC in a sealed-tube for 1 h. After cooling, the volatiles were evaporated under reduced pressure. The residue was treated with Et2O (5 ml) and filtered. The solid is compound 2 (0.4 g). The filtrate was concentrated to give compound 1 (0.31 g, 47%).
Method B: A solution of methyl (Z)-3-anilinoacrylate (3, 0.5 g) [1] in MeCN (2 ml) was heated under reflux for 5 h (or at r.t. for 15 d). The volatiles were evaporated under reduced pressure to give the compound 1 as a solid (0.36 g, 97.8%).
Data for compound 1:
M.p. 116.4-117.7 ºC (lit. [2]:118.9°C).
1H-NMR(CDCl3): 10.75 (d br, J = 13.2, 1H, NH), 7.76 (d, J = 13.2, 1H, H-5), 7.48 (d, J = 16.0, 1H, H-3), 7.34-7.40 (m, 2H, Ph), 7.11-7.16 (m, 1H, Ph), 7.06-7.10 (m, 2H, Ph), 6.19 (d, J = 16.0, 1H, H-2), 3.86 (s, 3H, OMe), 3.76 (s, 3H, OMe).
13C-NMR(CDCl3): 169.4 (C, COOMe), 168.8 (C, COOMe), 148.0 (CH, C-5), 142.4 (CH, C-3), 139.3 (C, Ph), 129.9 (2CH, Ph), 124.5 (CH, Ph), 116.7 (2CH, Ph), 110.7 (CH, C-2), 98.4 (C-4), 51.4 (OMe), 51.3 (OMe).
IR (cm-1, KBr): 2300-3200 br, 1694vs, 1655vs, 1629vs, 1615vs, 1597vs, 1590vs, 1439vs, 1371vs, 1309vs, 1261vs, 1223vs, 994s, 793s, 749vs.
CI-MS (NH3): 262 (M+H+, 6), 261 (M+, 1), 115 (3), 106 (2), 98 (6), 94 (100), 93 (34), 77 (49), 73 (38).
References
1. Huisgen, R.; Herbig, K.; Siegl, A.; Huber, H. Chem. Ber. 1966, 99, 2526.
2. Bottomley, W. Tetrahedron Lett. 1967, 8, 1997.
Sample availability: available from MDPI. MDPI ID 13337.
©1999 MDPI. All rights reserved. Molecules
website www.mdpi.org/molecules