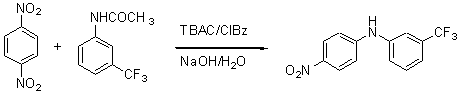
Departamento de Química y Física, Universidad Nacional de Río Cuarto, Agencia Postal Nro 3, 5800 Río Cuarto, Argentina. Tel. +358 4676157, Fax +358 676233, E-mail: [email protected]
Received: 25 November 1999 / Accepted: 15 December 1999 / Published: 24 January 2000
Keywords: diphenylamine, PTC, nitrobenzene, trifluoromethyl.
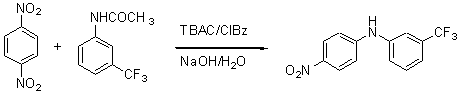
The general part of the experimental section and the purposes of this synthesis have been present elsewhere [1]. A solution of 1,4-dinitrobenzene (168 mg, 1.00 mmol), 3-acetamidobenzotrifluoride (495 mg, 2.44 mmol) and tetrabutylammonium chloride (TBAC, 69 mg, 0.25 mmol) in chlorobenzene (ClBz, 5 mL) was stirred for 10 min at 70 ºC. Then, 4 mL of 52.9 % w/w NaOH aqueous solution was added and the mixture was stirred for 16 hours at 70 ºC. The mixture was neutralized with ammonium chloride solution, washed with water and extracted with dichloromethane. The organic phase was dried with MgSO4, filtered and the solvent removed under reduced pressure. Flash column chromatography (silica gel, chloroform) afforded 74 mg of the pure N-(3-trifluoromethylphenyl)-4-nitroaniline.
M.p. 123-124 °C.
TLC (silica gel, chloroform): Rf 0.26
FT-IR (KBr): 3382 (NH), 3092 (CAr-H), 1602, 1512 (NO2), 1315 (NO2), 1304, 1108.
1HNMR (CDCl3): 6.37 (bs, 1H, NH); 7.01 (d, 2H, J=8.7Hz); 7.44 (m, 4H), 8.17 (d, 2H, J=8.7Hz).
MS (m/z): 282.1 (M+).
Anal. Calcd. for C13H9F3N2O2: C 55.33, H 3.21, N 9.93; found C 55.45, H 3.17, N 9.98.
Acknowledgment: This research was supported by Consejo Nacional de Investigaciones Científicas y Técnicas of Argentina and Fundación Antorchas.
Reference
1. Durantini, E. N.; Chiacchiera, S. M.; Silber, J. J. Synth. Commun. 1996, 26, 3849-3858.
Sample Availability: Available from the authors.
©1999 MDPI. All rights reserved. Molecules
website www.mdpi.org/molecules/