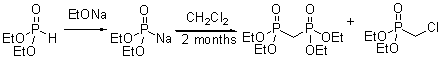
Methane-diphosphonic Acid Tetraethyl Ester
Didier Villemina and Frédéric Thibaultb
ISMRA, 6 boulevard Maréchal Juin, F-14050 Caen, France; a) UMR 6507 CNRS, E-mail: [email protected] b) UMR 6506 CNRS
Received: 16 December 1999 / Accepted: 17 January 2000 / Published: 24 January 2000
Keywords: phosphonate, bioactive compound, material, alkene precursor.
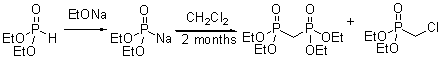
Methanediphosphonic acid tetraethyl ester
is a useful intermedaite for the synthesis of phosphonoalkenes [1] or diphosphonoalkenes
[2]. Methanediphosphonic acid tetraethyl ester is also a precursor of bioactive
compounds [3] and hybrid organic-inorganic materials [4]. Many synthetic
methods are available in the literature [5],
but none of them is very convenient and commercial methanediphosphonic
acid tetraethyl ester [6] remains expensive. We have improved the method
described by Horni [7]. Although our method is long (a 60 day reaction
at ambient temperature), the yield is excellent (89%) and the method is
simple and convenient in comparison with other methods of literature.
A solution of of sodium ethoxide was prepared
by adding sodium metal (3 mol, 69 g) in portions to absolute ethanol (900
ml). Diethyl phosphite (3 mol, 386ml) was added with stirring, and the
mixture stirred for 1h at ambient temperature. The excess of alcohol was
evaporated using a rotary evaporator. Dichloromethane (1000 ml) was added,
and the mixture was agitated for 2 months in a closed bottle. The mixture
was washed with water (500 ml) and the organic phase was dried over magnesium
sulfate. The solvent was evaporated, and the product was obtained as a
colourless liquid (89% yield) by distillation [Bp : 128 °C(0.02)].
RN : 1660-94-2
1H NMR (CCl4): 1.35 (t, J = 7 Hz, 12 H, P-O-C-CH3) 2.45 (t, J = 21 Hz, 2 H, P-CH2-P) 4.15 (dq, J1 = J2 = 7 Hz, 8 H, P-O-CH2).
13C NMR (CDCl3): 16.4 (P-O-C-CH3), 25.7 (t, J = 137 Hz, P-C-P) 62.55 (P-O-C).
31P NMR (CDCl3): 19.05.
IR (film) : 1250 (P=O), 1020 (P-O-C), 960, 820.
References
| 1. | Cardogan, J. I. G. In Organophosphorus reagents in Organic Synthesis, Academic Press, London, New York, 1979. Johnson A. In Ylid Chemistry, Academic Press, New York, 1966. Wadsworth,W. S., Jr. Organic Reaction 1978, 25, 73. Villieras, J.; Rambaud, M.; Kirschleger, B. Phosphorous and Sulfur, 1983, 14, 385. Villieras, J.; Rambaud, M. Synthesis, 1982, 924 and 1983, 300. Villieras, J.; Rambaud, M.; Graff, M. Tetrahedron Lett., 1985, 26, 53; Villemin, D.; Puciova-Sebova, M.; Toma, S.; Hachemi, M. Phoshorus, Sulfur, Silicon 1996, 113, 131. |
| 2. | Lehnert, W. Tetrahedron 1974, 30, 301. |
| 3. | Bijvoet, O.L.M.; Fleisch, H.A.; Canfield, R.E.; Russell, R.G.G. In Bisphosphonate on Bones, Eds Elsevier, Amsterdam, 1995. |
| 4. | Alberti, G. NATO ASI, Ser. C 1993, 179-90. Alberti, G.; Costantino, U.; Vivani, R.; Zappelli, P.; Rossodivita, A.; Bassignani, L. Eur. Pat. Appl. 1992, EP 469, 682 (C.A., 116: 263335j). |
| 5. | Czekanski, T.; Gross, H.; Costisella, B. J. Prakt. Chem. 1982, 324, 537. Chung, S.-K.; Moon, S.-H. Carbohydr. Res. 1994, 260, 39. Teulade, M.-P.; Savignac, P.; Aboujaoude, E.E.; Lietge, S.; Collignon, N. J. Organomet. Chem. 1986, 304, 283. Balczewski, P.; Mikolajczyk, M. Synthesis1995; 392 . Ezquerra, J.; Yruretagoyena, B.; Moreno-Manas, M.; Roglans, A. Synth. Commun. 1995, 25, 191. |
| 6. | Commercially available from Fluka, Lancaster Synthesis Ltd., Aldrich. External Access ID 359181 |
| 7. | Hormi, O.E.O.; Pajunen, E. O.; Avall, A.K. C. Pennanen, P. Synth.Commun. 1990, 12, 1865. |
Sample Availability: Available from the authors and from MDPI.
©2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/